To determine the equivalence of tranexamic administration topically, intravenously or the combination of both routes are equivalent in reducing blood loss after primary total hip arthroplasty.
Material and methodsIn this prospective study, we divided 285 THA into four groups. Group A received 2,5 g of intraarticular topical TXA, Group B did not receive TXA, Group C received 1 g intravenous TXA and group D a combination of topical and intravenous TXA. The main outcome was blood loss according to Nadler's formula and total hemoglobin.
ResultsBlood loss was significantly greater in Group B (Group A. 797,13 mL; Group B: 1308,24; Group C: 986,30 and Group D: 859,09 mL; P < ,01) with no differences between the other groups. Hemoglobin loss was greater in Group B (11.81, 19.46, 14.52 and 12.78 respectively, P < .001). Two patients (3,1%) were transfused in Group A, 4 (5,3%) in Group B, 3 (3,4%) in Group C, and 1 (1,8%) in Group D (P = ,75). The mean reduction in hemoglobin at 48 h was less in the topical Group (P < ,05). In comparison with Group B the mean reduction in hemoglobin after 48 h was greater (P < ,001) and we can also see a longer hospital stay (P < ,001). One patient in Group A presented pulmonary thromboembolism 72 h after surgery, which was resolved without complications.
ConclusionsThe administration of tranexamic acid topically, intravenously or in combination in primary total hip arthroplasty, is a simple and safe procedure that provides equivalent reductions in hemoglobin and blood loss.
Determinar la equivalencia de la administración de ácido tranexámico (TXA) tópico, intravenoso o combinado en la reducción de la pérdida sanguínea tras la artroplastia de cadera primaria.
Material y métodosEstudio prospectivo de 285 artroplastias primarias de cadera divididas en cuatro grupos. El grupo A recibió 2,5 g de TXA tópico, grupo B no recibió TXA, grupo C 1 g TXA intravenoso y grupo D administración combinada. La variable principal fue la pérdida sanguínea aplicando la fórmula de Nadler y de hemoglobina total.
ResultadosLa pérdida sanguínea fue significativamente mayor en el Grupo B (Grupo A: 797,13 mL, grupo B: 1308,24, grupo C: 986,30 y Grupo D: 859,09 mL; P < ,01) sin observarse diferencias entre los otros 3 grupos. La pérdida de hemoglobina fue mayor en el grupo B (11,81, 19,46, 14,52 y 12,78 respectivamente; P < ,001). Se transfundieron 2 pacientes (3,1%) en el grupo A, 4 (5,3%) en el grupo B, 3 (3,4%) en el grupo C y 1 (1,8%) en el grupo D (P = ,75). La reducción media de la hemoglobina a las 48 horas fue mayor en el grupo B (P < ,001) así como una estancia hospitalaria más larga (P < ,001). Un paciente del Grupo A presentó un tromboembolismo pulmonar a las 72 horas de la cirugía que se resolvió sin complicaciones.
ConclusionesLa administración de ácido tranexámico por vía tópica, intravenosa o combinada proporciona reducciones equivalentes en la pérdida de sangre y hemoglobina tras la artroplastia total primaria de cadera comparada con la no administración.
Total hip arthroplasty is associated with a perioperative blood loss of approximately one litre,1 which may lead to postoperative anaemia and the need for transfusion.2
Many blood saving strategies have been used, including controlled hypotension, regional anaesthesia and autologous blood transfusion, but they have not been demonstrated to be completely effective and consequence-free.2 In recent years, the use of tramexanic acid (TXA) has become a popular drug for reducing blood loss and the risk of transfusion after total hip arthroplasty, without increasing the risk of thromboembolic complications.2,3
TXA is a derivative of the synthetic amino acid lysine which inhibits the attachment of fibrin to the plasminogen, thus preventing the deterioration of the fibrin blood clot by the plasmin.4 The efficacy of topical or intravenous TXA has been demonstrated in the reduction of blood loss and drainage blood loss in total hip and knee arthroplasty.2 Intravenous administration achieves a rapid TXA transfusion in the synovial fluid of the target joint but there are contraindications such as a previous history of pulmonary thromboembolism, deep vein thrombosis or ischaemic events such as acute myocardial infaction.5 For its part, topical administration provides maximum concentration of TXA in the surgical site associated with a minimal systemic effect, thus reducing possible systemic adverse effects.6,7
The aim of this study was to determine whether topical, intravenous or a combination of both routes were equivalent in the reduction of blood loss, compared with the non-administration of TXA. A secondary aim was to determine its impact in the need for transfusion, hospital stay and the rate of complications.
Material and methodsThis prospective, non-randomised study was approved by our ethics committee (PI:2626).
Patients diagnosed with coxarthrosis and who underwent primary total hip arthroplasty between April 2015 and May 2017 were included. All patients who were hypersensitive to TXA, had a previous history of thromboembolism, hypercoagulability or a disease of the heart or brain which necessitated dicoumarinic anticoagulant treatments were excluded. Data was collected from a total of 285 primary arthroplasties of the hip in 266 patients who were included in blood-saving protocols with the administration of TXA in our centre. We distinguished between 4 patient groups according to TXA administration route. Group: A: topical (65 hips), group B: no TXA administration (76 hips), group C: intravenous (88 hips) and group D: combined topical and intravenous administration (56 hips). The TXA administration route was modified in our centre during this study period with the result that, in 2015, it was only administered intravenously whilst in successive years the topical route or both routes were chosen, depending on the preferences of the surgical team. Patients were included in the different study groups successively, not randomly.
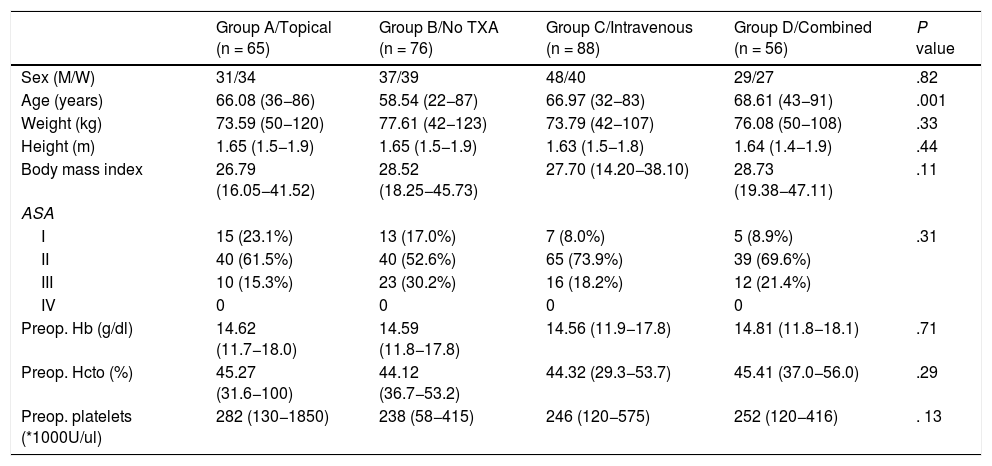
Demographic and preoperative patient data were collected including age, sex, classification of the physical state of the American Society of Anesthesiologists (ASA), weight, height, body mass index, and preoperative analytical levels of haemoglobin (Hb), haematocrit and platelet counts of each group (Table 1).
Demographic characteristics and preoperative levels.
| Group A/Topical (n = 65) | Group B/No TXA (n = 76) | Group C/Intravenous (n = 88) | Group D/Combined (n = 56) | P value | |
|---|---|---|---|---|---|
| Sex (M/W) | 31/34 | 37/39 | 48/40 | 29/27 | .82 |
| Age (years) | 66.08 (36−86) | 58.54 (22−87) | 66.97 (32−83) | 68.61 (43−91) | .001 |
| Weight (kg) | 73.59 (50−120) | 77.61 (42−123) | 73.79 (42−107) | 76.08 (50−108) | .33 |
| Height (m) | 1.65 (1.5−1.9) | 1.65 (1.5−1.9) | 1.63 (1.5−1.8) | 1.64 (1.4−1.9) | .44 |
| Body mass index | 26.79 (16.05−41.52) | 28.52 (18.25−45.73) | 27.70 (14.20−38.10) | 28.73 (19.38−47.11) | .11 |
| ASA | |||||
| I | 15 (23.1%) | 13 (17.0%) | 7 (8.0%) | 5 (8.9%) | .31 |
| II | 40 (61.5%) | 40 (52.6%) | 65 (73.9%) | 39 (69.6%) | |
| III | 10 (15.3%) | 23 (30.2%) | 16 (18.2%) | 12 (21.4%) | |
| IV | 0 | 0 | 0 | 0 | |
| Preop. Hb (g/dl) | 14.62 (11.7−18.0) | 14.59 (11.8−17.8) | 14.56 (11.9−17.8) | 14.81 (11.8−18.1) | .71 |
| Preop. Hcto (%) | 45.27 (31.6−100) | 44.12 (36.7−53.2) | 44.32 (29.3−53.7) | 45.41 (37.0−56.0) | .29 |
| Preop. platelets (*1000U/ul) | 282 (130−1850) | 238 (58−415) | 246 (120−575) | 252 (120−416) | . 13 |
A total of 145 patients (50.9%) were men and 140 women (49.1%) with a mean age of 64.84 ± 12.75 years.
The TXA administration protocol was as follows. The intravenous guideline consisted in the injection of 1.5 g of TXA in bolus prior to surgical incision and the other dose of 1.5 g in continuous infusion during surgery. The guideline for the topical route consisted in intra-articular administration of 2.5 g of TXA with no dilution after the closing of the fascia.
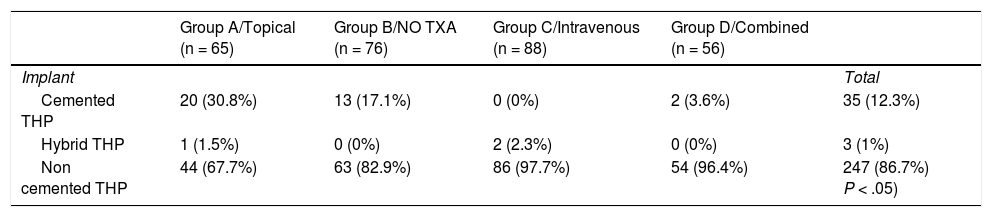
All patients were operated on under spinal anaesthesia and using the posterior hip approach. The implant choice decision depended on the patient’s age, the bone characteristics and the surgeon’s preferences (Table 2). No postoperative draining was used. All patients received intravenous antibiotic prophylaxis for 24 h and thromboembolic prophylaxis with low molecular weight heparin of daily subcutaneous administration for 6 weeks. Sitting was initiated after 24 h and standing with crutches 24−48 h after surgery. Analytic controls were performed after 24 and 48 h with determination of Hb and platelet levels. Transfusion was specified by the orthopaedic surgeon if the Hb level was <7.0 g/dL, and for those patients with a pre-existing cardiovascular disease the transfusion was specified if they presented with an Hb of 8 g/dL or under, together with accompanying medical conditions. A restrictive transfusion strategy of the Patient Blood Management (PBM) programmes8 was adhered to.
Type of prosthesis implanted.
| Group A/Topical (n = 65) | Group B/NO TXA (n = 76) | Group C/Intravenous (n = 88) | Group D/Combined (n = 56) | ||
|---|---|---|---|---|---|
| Implant | Total | ||||
| Cemented THP | 20 (30.8%) | 13 (17.1%) | 0 (0%) | 2 (3.6%) | 35 (12.3%) |
| Hybrid THP | 1 (1.5%) | 0 (0%) | 2 (2.3%) | 0 (0%) | 3 (1%) |
| Non cemented THP | 44 (67.7%) | 63 (82.9%) | 86 (97.7%) | 54 (96.4%) | 247 (86.7%) P < .05) |
The P value refers to the statistical comparison through the Chi squared test.
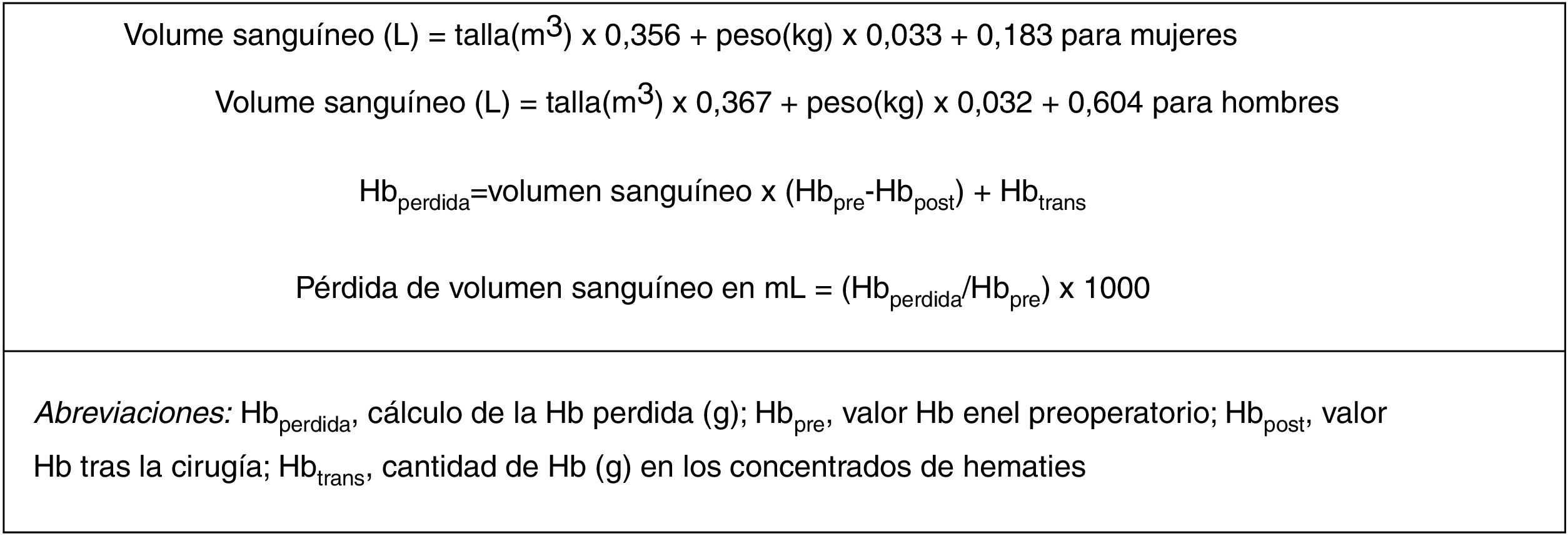
The main resulting variable in this study was blood loss, which was calculated from the Hb level, the concentration of preoperative Hb and the concentration of postoperative Hb, the number of transfusions and the estimated blood volume. The volume of blood was determined by sex, body mass index and height, based on the Nadler9 formula (Fig. 1). Secondary results included the need for transfusion, the number of units of blood transfused, hospital stay duration and rate of complications.
The Student’s t-test, Pearson Chi-square and Mann-Whitney tests were used to determine whether the differences between the groups were significant. The main findings regarding blood loss in milliliters (mL), and the loss of Hb were evaluated using the ANOVA test, and the secondary results were compared using the chi-squared statistical test. Significance was established at 5% (P < .05) and correction of errors due to multiple tests, for analysis subsets was achieved using the Bonferroni method. All analyses were carried out in SPSS 11.5 (SPSS Inc, Chicago, IL, United States).
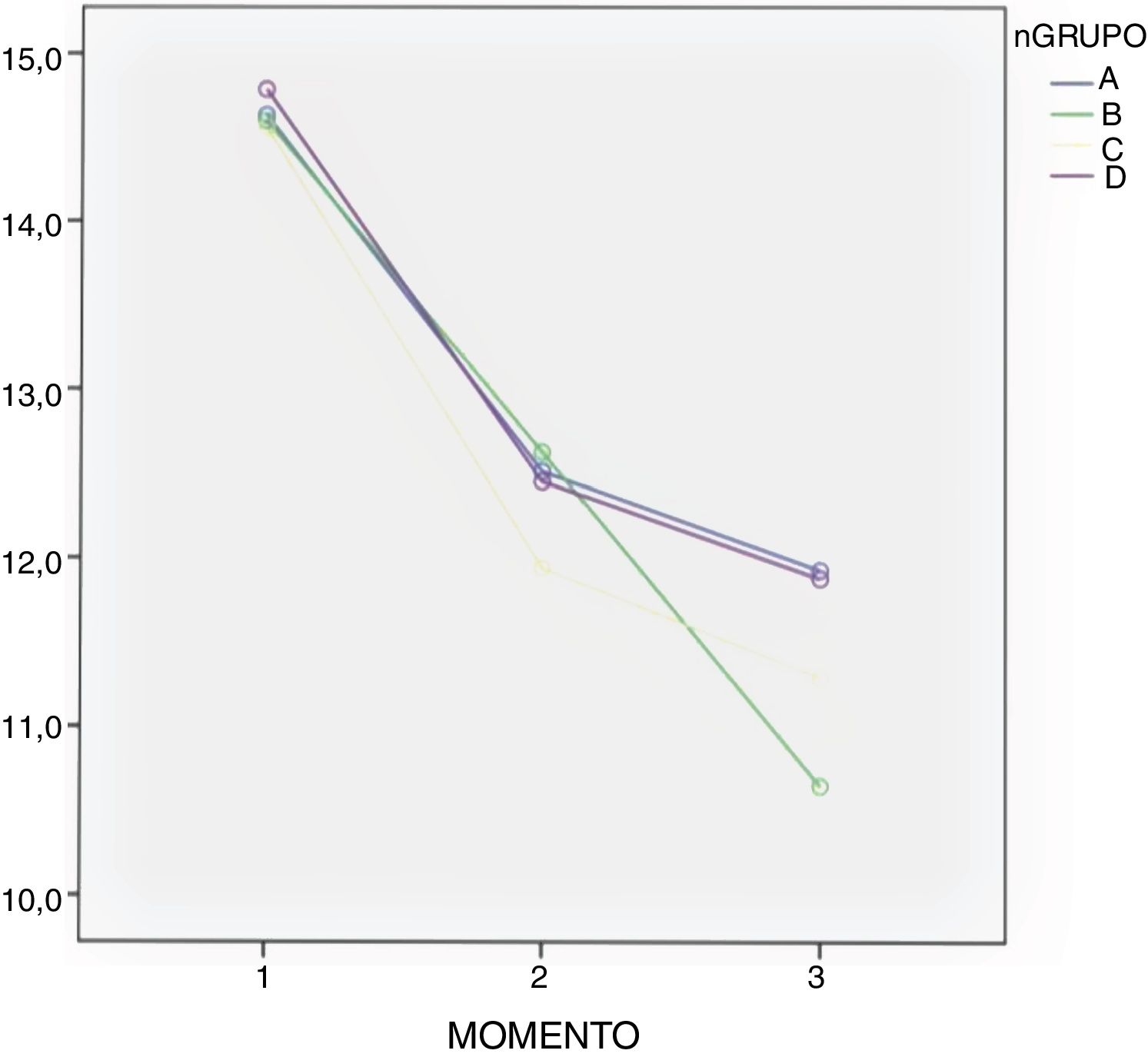
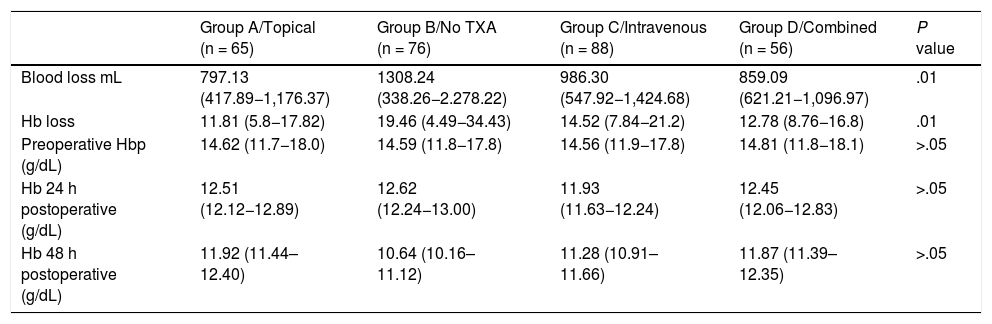
ResultsClinical resultsMean blood loss measured in mL was 797.13 ± 379.24 in group A; 986.30 ± 438.38 in group C; 859.09 ± 237.88 in group D, whilst in the control group (group B) it was 1,308.24 ± 969.98 (P < .01). A significantly lower Hb loss was observed in the 3 groups where TXA had been administered (Table 3).
Blood loss measured in mL and loss of Hb, calculated according to the Nadler formula; preoperative Hb levels and postoperative levels measured after 24 and 48 h.
| Group A/Topical (n = 65) | Group B/No TXA (n = 76) | Group C/Intravenous (n = 88) | Group D/Combined (n = 56) | P value | |
|---|---|---|---|---|---|
| Blood loss mL | 797.13 (417.89−1,176.37) | 1308.24 (338.26−2.278.22) | 986.30 (547.92−1,424.68) | 859.09 (621.21−1,096.97) | .01 |
| Hb loss | 11.81 (5.8−17.82) | 19.46 (4.49−34.43) | 14.52 (7.84−21.2) | 12.78 (8.76−16.8) | .01 |
| Preoperative Hbp (g/dL) | 14.62 (11.7−18.0) | 14.59 (11.8−17.8) | 14.56 (11.9−17.8) | 14.81 (11.8−18.1) | >.05 |
| Hb 24 h postoperative (g/dL) | 12.51 (12.12−12.89) | 12.62 (12.24−13.00) | 11.93 (11.63−12.24) | 12.45 (12.06−12.83) | >.05 |
| Hb 48 h postoperative (g/dL) | 11.92 (11.44–12.40) | 10.64 (10.16–11.12) | 11.28 (10.91–11.66) | 11.87 (11.39–12.35) | >.05 |
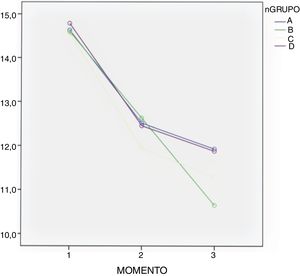
In all groups a drop in Hb levels was observed, measured 24 and 48 h after surgery with no significant differences according to the Bonferroni multiple comparison test between the groups (Table 3 and Fig. 2).
A total of 10 patients received transfusions, 2 in group A (3%), 3 in group C (3.4%), one in group D (1.8%) and 4 in the control group (group B) (5.3%), with no significant differences being found between the groups (P = .748).
Mean hospital stay was 5.4 ± 3.1 days and was significantly higher P < .001) in the control group (group B) 6.85 + 4.03 days. In group A it was 5.65 + 2.88 days, in group C 5.59 + 2.65 days and in group D 5.43 + 3.19 days.
Only one patient in group A had a pulmonary embolism which occurred 72 h after surgery and was resolved without complication.
DiscussionThe administration of TXA in total hip arthroplasty has become popular in recent years since it has been shown to be an effective strategy for reducing blood loss and the need for blood transfusions.2,3 We present the results of the administration of TXA by different routes in 285 primary hip arthroplasties.
Administration route continues to be under debate. Many clinical trials compare the oral route with the combination of topical and intravenous routes,3 as detailed in a meta-analysis performed by Gianakos et al. in 2018.2 Most studies support the use of combined therapy against monotherapy, but no study analyses combined therapy compared with topical administration. Luo et al. compared the administration of topical, oral and intravenous TXA, concluding that the oral route was equivalent to the other routes of administration in efficacy as a method of blood control.10 These same conclusions were supported by other authors,7 and in our study we did not find any differences in blood loss and in Hb between the 3 groups in which TXA was administered. We also observed a mean drop of Hb in all groups, although it was higher in the control group (group B) as detailed in Fig. 2. A hypothesis exists that the therapeutic concentration in the haemorrhage site may be lower when intravenous administration is used for TXA dissemination in systemic circulation,2 and as a result the topical route could have higher efficacy in control of bleeding. However, there are no studies to show this theoretical advantage of topical TXA administration.
Regarding the administration moment and dosage, there are different protocols with doses which vary between .5 and 3 g of TXA.2,7,11–14 In our centre the intravenous protocol consisted in the administration of 1.5 g of TXA in bolus prior to incision and 1.5 g of TXA in continuous infusion during surgery, and when we chose to apply it topically 2.5 g of TXA was administered without diluting on finalization of surgery prior to closure, thus achieving maximum TXA concentration in the hip joint volume.
Due to its antifibrinolytic role TXA has been described as theoretically presenting the possibility of increasing the risk of thromboembolism.2 It has not been demonstrated that the risk of venous thromboembolism increases with TXA, but it is advised that the patient’s history and risk factors be considered when choosing administration route and dosage.2 In our study only one patient from the topical administration route presented with pulmonary thromboembolism 72 h after surgery and this was resolved with medical treatment. This event occurred in a patient with no associated PTE risk factors.
In the meta-analyses performed by Gianakos et al.2 it was shown that TXA reduced the number of transfusions per patients by .78 units. In our study we did not find there were differences regarding the need for transfusion compared with the group of no TXA administration. However we did find differences in hospital stay duration, which was longer in the group who were not administered TXA with a mean of 6.85 ± 4.03 days compared with the groups where it was administered topically (5.65 ± 2.88 days), intravenously (5.59 ± 2.65 days) and where administration was combined (5.43 ± 3.19 days).
LimitationsThis study has a series of limitations. First, because it is a descriptive study its level of scientific evidence is not high. Another limitation is the fact that the possible haemodilution effect on blood loss calculation was not considered. Also, due to the low frequency of transfusions and thromboembolic events, this study does not have sufficient power to find differences between groups.
ConclusionsThe administration of TXA topically, intravenously or in combination in primary hip arthroplasty provides equivalent reductions in blood loss and Hb after primary total hip arthroplasty, compared with non-administration. It is a simple, safe procedure which we believe should form part of the routine procedure in perioperative management of the patient who will undergo a hip arthroplasty. It is the surgical team who, based on the patient’s comorbidities and characteristics, will determine the best administration route.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Gómez-Luque J, Cruz-Pardos A, Garabito-Cociña A, Ortega-Chamarro J, García-Rey E. El ácido tranexámico tópico, intravenoso y su uso combinado son equivalentes en la reducción de la pérdida sanguínea tras la artroplastia total de cadera primaria. Rev Esp Cir Ortop Traumatol. 2021;65:349–354.