Laundry detergents capsules (CDR, for its acronym in Spanish) in compact powder format (tabs), powder and liquid (caps), or liquid (pods), have experienced great marketing development since its release in Spain. The objective is to describe the consultations registered in the Spanish Control Center related to CDR exposure.
Material and methodsA retrospective study of poison centre calls due to exposure to laundry detergents in capsule format between January 1st, 2008 and December 31st, 2014. The information has been obtained from the Spanish Poison Centre database (Servicio de Información Toxicológica).
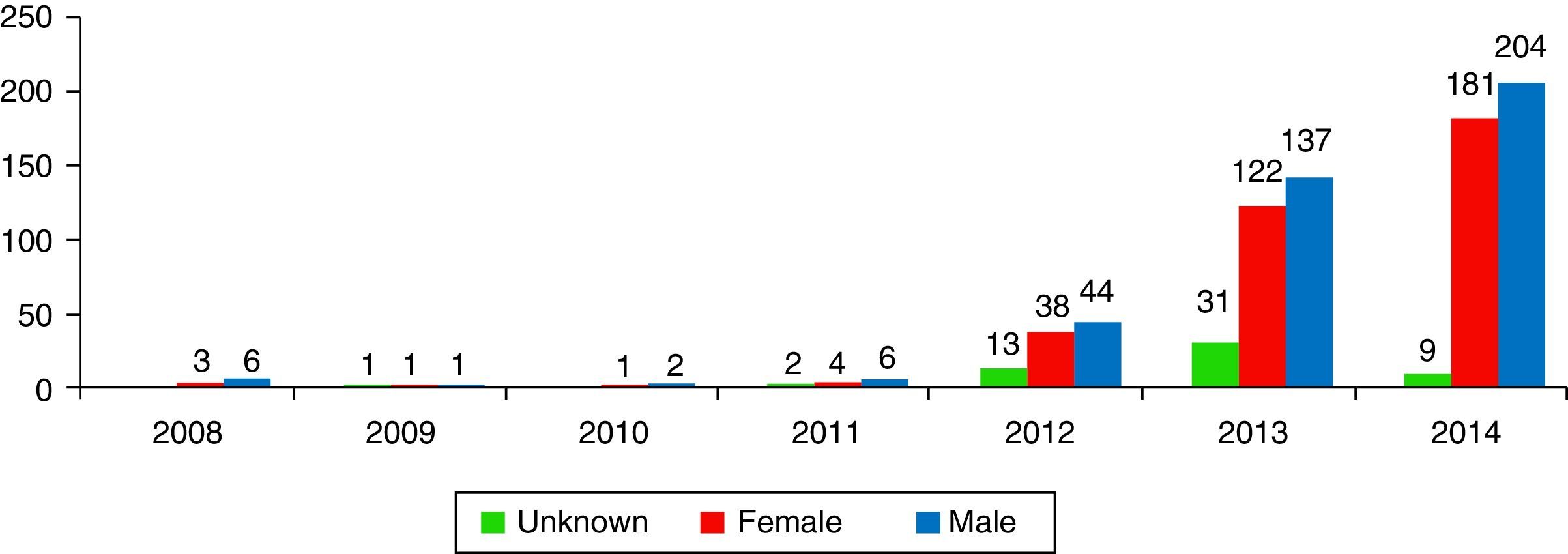
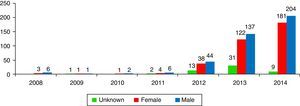
ResultsA total of 806 cases that implicated CDR were recorded, accounting 9 cases in 2008 and rising to 398 in 2014. The profile of the intoxicated/victim corresponds to a 19–24 month-old male who swallows the contents of the capsule.
ConclusionsThere has been such an increase of queries related to CDR exposure that, due to an increased risk of clinical effects, we have to insist about the need for this type of product to be kept out of children's reach.
Las cápsulas de detergente de ropa (CDR), tanto en forma de polvo compacto, como en polvo/líquido o como líquido han experimentado un gran auge desde su comercialización en España. El objetivo es describir las consultas por exposición a CDR que se han registrado en el centro antitóxico español de referencia.
Material y métodosEstudio observacional de casos de consultas telefónicas por exposiciones a CDR entre el 1 de enero de 2008 y el 31 de diciembre de 2014. La información se obtuvo de la base de datos del Servicio de Información Toxicológica.
ResultadosSe registraron 806 casos en los que el producto implicado eran CDR, pasando de 9 casos en el año 2008 a 398 en el año de 2014. El perfil del intoxicado corresponde a un varón de entre 19 y 24 meses que ingiere el contenido de la cápsula.
ConclusionesSe ha producido tal incremento de las consultas por exposiciones a CDR que, debido a un mayor riesgo de efectos clínicos, es importante incidir en la necesidad de mantener este tipo de producto fuera del alcance de los niños.
Laundry detergent capsules (LDCs) are a new way of packaging laundry detergent that arrived on the European market in 2001 with the aim of replacing liquid detergents. In spite of their higher cost, consumption of capsules has increased greatly due to their being easier to transport, store, use and measure.
Spanish consumers can buy them in three formats. In 2004, the solid powder pill format appeared (±21g), known as tabs, which dissolve immediately. This format is currently disappearing at the industry's initiative. The content of tabs is granular and hygroscopic, and it binds easily to the oropharyngeal and oesophageal mucosa if swallowed; moreover, their more alkaline pH (10.5–12 in a 1% solution) can generate liquefactive lesions in the digestive tract if contact is prolonged. In September 2010, the caps format arrived with a liquid/powder interior and an approximate weight of 19–20g. Caps remain intact so long as they are dry, but they dissolve completely in water in less than 2min. In 2012 the liquid pod format appeared. (Pods is the term that is used in the U.S., and which marketing teams have applied for all markets.) Pods are small soft packets or single-dose bags wrapped in an alcohol/polyvinyl film or membrane that is highly water soluble. They have an approximate volume of 28ml and weigh ±23–29g.1,2 The wrapping is designed to degrade quickly and homogeneously when it comes into contact with liquid, regardless of the number of chambers in the pod (1–3). Distributing the pod's components into different chambers makes it more stable (Fig. 1). The main ingredients in a typical laundry detergent are anionic and nonionic surfactants, plus sequestrants, optical brighteners, anti-redeposition agents, soaps, perfumes, enzymes and preservatives.3 While the quantitative composition of detergents does not vary from one format to another, in capsule formats (especially pods) the concentration range is 2.5–5% higher. The higher the concentration of the surfactants, the greater the cytotoxicity they can produce. While powder detergents contain up to 20% anionic surfactants, in capsules this increases to 50%. The purpose of sequestrants is to achieve optimal cleaning in spite of hard water. They are high-cost components that come in concentrations under 1%. Phosphates are avoided since they are harmful to the environment. There is no water in the composition because it would attack the integrity of the membrane. There are various alcohols that act as nonionic surfactants (glycol, propylene glycol, alcohol ethoxylates C12-14 and C14-15) which represent up to 34% of the volume. These detergent formats do not contain sodium hydroxide. The pH is neutral (7–9) in laundry detergents.3
Since the format is attractive for children due to the brighter colours and texture, they may mistake LDCs for candies or toys. Children may be tempted to play with them, squeeze them or pop them, or even put them in their mouths, which may lead to toxic contact or poisoning. The greatest risk is the capsule being ingested, with digestive symptoms and potential breathing complications.
The goal is to review the cases of toxic exposure from LDCs in Spain based on the reference information from the Spanish poison centre.
Material and methodsObservational study of cases of telephone calls for exposures to LDCs. The source of information was the Spanish Poison Information Service (SIT), which is part of the Madrid Department of the National Toxicology and Forensic Sciences Institute (INTCF). The SIT has acted as a poison centre since 19714 and it has a medical/poisoning phone line that is free, anonymous and confidential with its emergency line (915 620 420) which is open 24hours a day, 365 days a year. The continuity of this help line means that cases of exposure to toxic substances or possible poisonings can be evaluated and tracked. The phone line receives calls from throughout Spain, and calls come from healthcare professionals of all levels and from people with no specific healthcare training.
The main tool the SIT uses to perform its tasks is a database that it has prepared internally with over 179,000 files of products that may be sources of poisoning, and which include both active ingredients and products on the market in Spain. A toxicology study has been conducted for each file that makes it possible to provide an immediate response regarding possible poisonings from any of the products communicated to our service. The compositions of multiple commercial products are reported to the INTCF in compliance with the current legal regulations.5,6 In 1989, the National Consumption Institute (the current Spanish Agency of Consumption, Food Security and Nutrition, AECOSAN), the INTCF and the Association of Detergent and Cleaning, Maintenance and Accessory Companies (ADELMA), signed a collaboration agreement to promote consumer safety, in order to publicise the existence and functions of the SIT.
For this study, the calls were analysed that were recorded in the SIT from 01/01/2008 to 31/12/2014. 17% of the calls were due to toxic exposures to domestic cleaning products, and 95.2% affect minors under 14. All of the calls were reviewed where, according to the information provided by the person requesting information, the victim may have experienced toxic exposure to a laundry detergent, and where enough information could be gathered to create a poison centre record or note in the database, from which the information was taken for statistical purposes. These calls were classified, regardless of the commercial brand, as “laundry detergent”. After this, the calls were selected where there may have been toxic exposure to an LDC. The SIT does not have a specific classification system for these types of formats, and the category includes detergents in compacted format (tabs), liquids (pods) and liquids/powders (caps). Calls were excluded where the purpose of the call was something other than possible toxic contact or poisoning or where there were cases of exposure in animals.
The types of exposure were catalogued and grouped together into ingestion, inhalation, ocular contact, cutaneous contact, and multiple types of contact (combinations of two or more types, with neither predicating a priority classification). Aspiration was not included as a type of contact.
Results1% of the 704,095 calls the SIT received from 01/01/2008 to 31/12/2014 (n=7317) were due to potentially toxic exposure to laundry detergent. More specifically, of these 7317 cases, 11.1% (n=813) were exposures to LDCs, 35.7% (n=2611) were exposures to liquid detergent, 10% (n=735) were exposures to powdered detergent, 4.6% (n=333) were exposures to solid detergent, and 38.6% of the cases (n=2825) had no specific classification. 9 calls were received in 2008, while 394 were received in 2014 (Fig. 2).
The autonomous communities from which the most calls came were Andalusia (16.4%), Catalonia (16.4%), Valencian Community (14.9%) and Madrid (14.3%).
In 56.1% of the cases, the person requesting information was a healthcare professional to whom the poisoning victim came or was brought, while in 43.9% of the cases the calls came from people with no healthcare training.
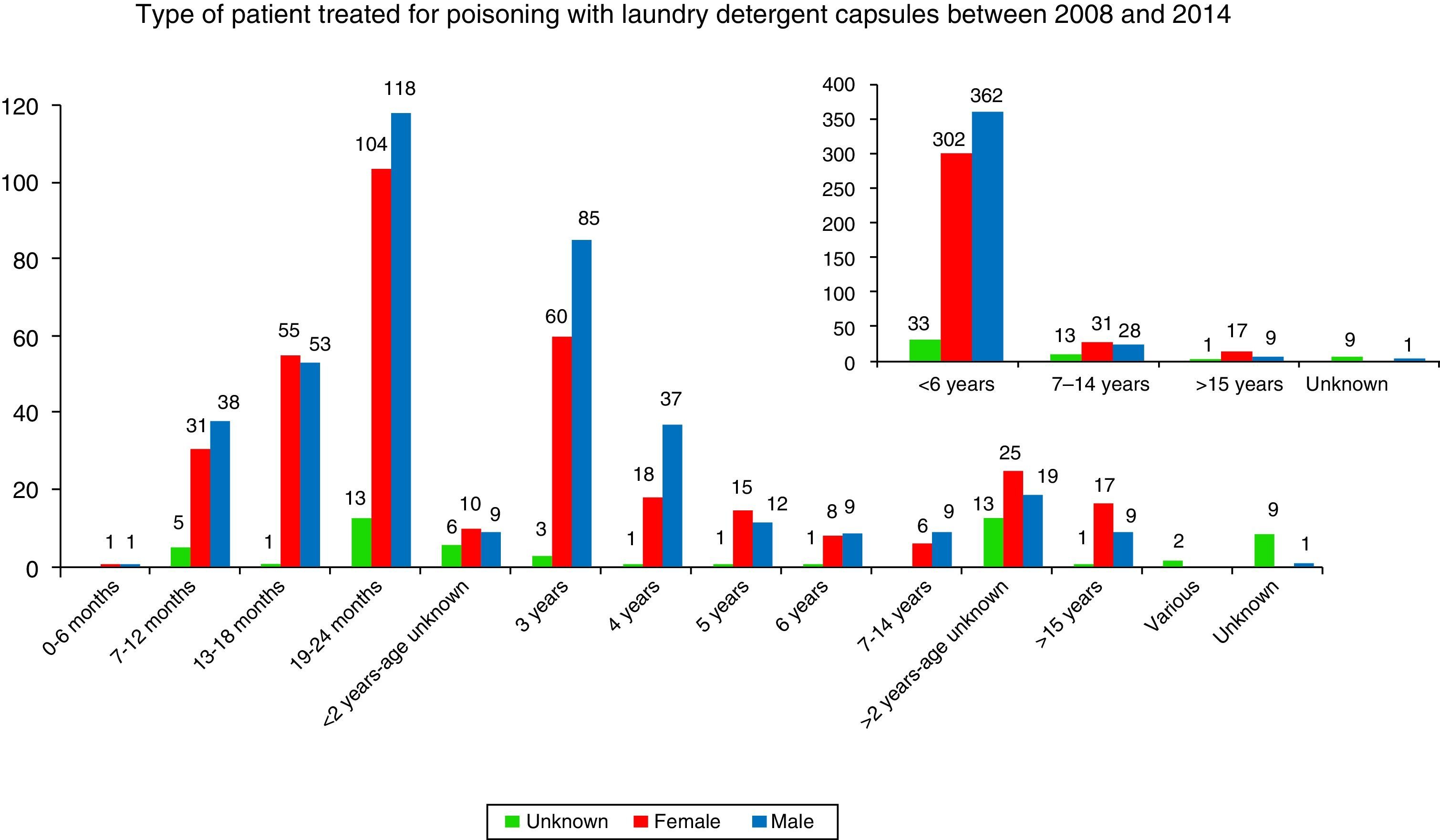
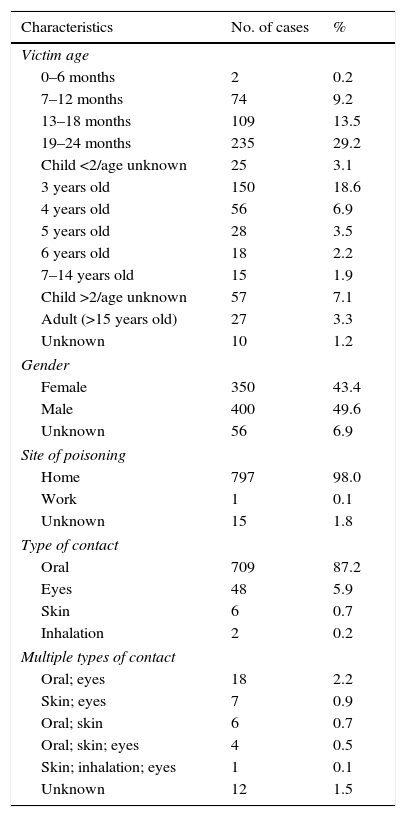
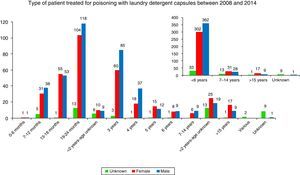
By age (Table 1), the exposures to LDCs were most frequent among 19–24 month-olds (29.2%), followed by 3-year-olds (18.4%), 13–18-month-olds (13.5%) and 4- to 6-year-olds (12.7%). Toxic exposures in adults only represented 3.3% of the total. In 1.2% of the cases the victim's age was unknown. By gender, 49.6% of the cases involved males and 43.4% involved females. The victim's gender was not known in 6.9% of the cases.
Characteristics of exposures to detergents in capsules.
| Characteristics | No. of cases | % |
|---|---|---|
| Victim age | ||
| 0–6 months | 2 | 0.2 |
| 7–12 months | 74 | 9.2 |
| 13–18 months | 109 | 13.5 |
| 19–24 months | 235 | 29.2 |
| Child <2/age unknown | 25 | 3.1 |
| 3 years old | 150 | 18.6 |
| 4 years old | 56 | 6.9 |
| 5 years old | 28 | 3.5 |
| 6 years old | 18 | 2.2 |
| 7–14 years old | 15 | 1.9 |
| Child >2/age unknown | 57 | 7.1 |
| Adult (>15 years old) | 27 | 3.3 |
| Unknown | 10 | 1.2 |
| Gender | ||
| Female | 350 | 43.4 |
| Male | 400 | 49.6 |
| Unknown | 56 | 6.9 |
| Site of poisoning | ||
| Home | 797 | 98.0 |
| Work | 1 | 0.1 |
| Unknown | 15 | 1.8 |
| Type of contact | ||
| Oral | 709 | 87.2 |
| Eyes | 48 | 5.9 |
| Skin | 6 | 0.7 |
| Inhalation | 2 | 0.2 |
| Multiple types of contact | ||
| Oral; eyes | 18 | 2.2 |
| Skin; eyes | 7 | 0.9 |
| Oral; skin | 6 | 0.7 |
| Oral; skin; eyes | 4 | 0.5 |
| Skin; inhalation; eyes | 1 | 0.1 |
| Unknown | 12 | 1.5 |
With regard to the type of contact with the product, most of the cases were oral (87.1%), followed by eye contact (6.0%), multiple contacts (4.5%), skin contact (0.7%), inhalation (0.2%) and unknown (1.5%).
The adverse health effects recorded by the SIT based on the type of contact were: vomiting, nausea, cough, oropharyngeal irritation, drooling, diarrhoea and drowsiness (oral contact); eye irritation, conjunctivitis and keratitis (eye exposure), and erythema and pruritus (skin contact).
DiscussionThis study provides the first data in Spain on poison calls due to exposure to or contact with laundry detergents in capsule format. The SIT did not record any notable changes in the last seven years in exposures to household cleaning products, and in particular to laundry detergents, until the new detergent formats began to be codified, at which point they rose from 1.1% of total poisonings (in 2008) to 1.9% (in 2013). In the U.S., domestic cleaning products represented 8.3% of the cases studied in 2001–2010, while they represented 18.3% in Spain from 2008–2014.7 In 2000, exposures to domestic use laundry detergents represented 5.7% of the cases recorded by US poison centres. In Spain, they were 2.7% of the cases in 2000. In 2014 there was an explosion in the number of cases recorded by the SIT, explained by more precise data gathering.
The percentage of minors is of powerful note (Fig. 3) and especially in minors under 2 (55.2%). The percentage of exposure to LDCs in children under 5 was 84.0%, significantly lower than in the United Kingdom8 (96.1%). While it is not as excessively high as in the UK, the percentage is very high and doubly concerning because it shows that the smallest children are able to access capsules to ingest them and because the child population is more sensitive to the effects of these products.
The 2009/10 Annual Report of the UK National Poisoning Information Service indicated that the household product that caused the most poisonings from ingestion in the country were liquid detergent capsules, and that the number of reported cases had doubled in the past five years. It also pointed to the secondary risks of eye contact with this type of detergents, of conjunctivitis and keratitis, which take up to 7–10 days to resolve.7,9–11
Since becoming available in Italy in 2010, caps have been catapulted into first place among ingested toxic substances.
The entry of caps into the US market in 2012 saw a monthly increase of 645.3% among children under 6 from March 2012 to April 2013.12
The cases in Spain involved vomiting, nausea, cough, oropharyngeal irritation and salivation (38.9%); eye irritation, conjunctivitis and keratitis (5.2%); erythema and pruritus (0.1%); and dizziness, headache, and drowsiness (0.4%). 42.1% of the patients remained asymptomatic, while in 12.5% of the cases the symptoms were not known. These figures are very similar to those recorded by the poison centres of the United Kingdom, where 96% of the cases occurred in children under 5 who ingested the product and had nausea (3.5%), vomiting (24.1%), cough (4.1%) and rash (1.7%).7,8 A rash from ingesting detergent (usually on the abdomen, buttocks, legs or arms) that resolves in 3–24h7,8 is difficult to explain. One possibility might be that this is related to prolonged skin contact with the content of the capsules (a child who did not immediately wash or take off clothes impregnated where the toxic event took place, or who was carried to the medical centre in such adverse conditions, which would be likely to cause contact with these body parts).
The sequestrants (ion chelators) in conventional detergent formulas may cause hypocalcaemia and hypomagnesemia, but only in cases where large quantities have been ingested.3 Single dose caps and pods have low sequestrant concentrations (<1%), which makes it unlikely for there to be electrolyte imbalances in Spain as occurred in the cases in the United States. This can also be related to the higher concentration of these preparations in the US market.
The Royal Hospital for Sick Children of Glasgow (UK) reported five cases of children under 2 who were admitted with vomiting, drooling and throat wounds (confirmed by endoscopy) after having accidentally ingested LDCs.13 These findings make sense because some brands add an alkali to their formula that once ingested increases the harmful effects to the arytenoids, epiglottis and oesophagus, the points where there is the most contact.
The clinical findings are unquestionable, but from our point of view, if the composition and formulation of domestic use LDCs with neutral pH is taken into account, the caustic effect cannot occur. The cases with digestive repercussions and the endoscopic findings may have been due to the laundry detergents being for professional or institutional use.
In the first semester of 2012, the poison centre associated with the Children's Hospital of Philadelphia (U.S.A.) received four cases of children with vomiting, diminished consciousness and respiratory distress after having ingested liquid from pods.14 In line with this increased clinical repercussion, the SIT recorded three cases of exposure to pods in 2013: an adult male who inhaled the product in a laboratory and had signs of dizziness and headache leading him to be sent to the health centre; and two cases of children who were sent to hospital for observation: a 13-month-old boy who had signs of drowsiness, and a 22-month-old girl who suffered from drowsiness and diarrhoea. The drowsiness could be explained by the presence of various alcohols (glycol, propylene glycol, alcohol ethoxylate C12-14 and C14-15).
This study is a good example of the social and healthcare value of the INTCF. In addition to its valuable assistance in the field of justice, the INTCF, and more specifically the SIT, performs the toxicological surveillance work inherent to all poison centres, and it lends its services to society, providing the necessary information for preventing poisoning and improving the safety of new products.15
As the poison centres in the United Kingdom have warned,7,8 due to the rapid increase in calls due to exposures to caps/pods, and the fact that a considerable percentage of them occur in children, strategies need to be developed for tracking and preventing future poisonings, and to get parents involved in ensuring that these products are stored safely. We therefore remain in contact with the competent healthcare authorities in order to persist with our toxicological surveillance criteria, with the goal of ensuring better control of new potentially poisonous products launched onto the market, and defining the appropriate preventive measures for improving the health of our population. This contact between institutions helps the AECOSAN remain periodically informed of the details of these types of exposures.
It is crucial to follow the appropriate preventive measures that are recommended for any type of cleaning product, and which in the specific case of LDCs entail a higher risk of poisoning for younger children. Storing and closing these products safely every time they are used is fundamental. In Spanish homes, these products are usually stored in the kitchen, since this is where the washing machine is most commonly installed, and it is therefore where these poisonings are most likely to occur. In northern European countries, the bathroom is the location with the highest toxic risk for detergents. Short shrift should not be given to efforts geared towards preventing exposure in children, especially in the most affected age group (<6). These efforts should focus on packaging formats that are safer, and on having labels that are clearer and more appropriate, with warnings of the possible risks. And as the fundamental pillar, focus should be placed on educating and training the population.
Therefore, the SIT works together with the ADELMA and the European detergent industry in its campaign to promote safe use (“Keep the capsules away from children”). In view of the various reports on toxic exposures gathered by the various poison centres, what the industry is doing–in a practically generalised manner and as a standard for boosting the safety of all the chemical products they sell in Europe, as of 01 June 2015, and with the possibility of a temporary extension to 31 December 2015–is adopting a series of measures:
- 1.
Changing storage packaging. It had previously been transparent, but now it is opaque to prevent the single-dose capsules from being visible (measure introduced for the first time in Italy in 2011). In addition to this, there is a visible warning that immediately draws attention reading “Keep out of the reach of children”.
- 2.
Labelling the storage box or bag with rhombus-shaped danger icons and rapid treatment measures in the event of exposure. The rhombus icons indicate the nature of the dangers related to using a hazardous product.
- 3.
Since it has a more complex closure, both hands need to be used in coordination with a certain amount of strength to open the package (in both box and bag package types). The safety closure of the package remains even after it has been opened and closed repeatedly. Its main purpose is to prevent children from opening the package.
- 4.
The film or membrane of the pod format has been changed, soaking it in a non-toxic repellent substance that is bitter (denatonium benzoate). When sucked on, the strong bitter flavour remains for up to six seconds, enough time for the pod to be effectively rejected due to its bad flavour. Using higher concentrations of the repellent does not necessarily mean greater safety.
- 5.
The film has been made more resistant to dissolution (with homogeneous degradation), pressure or extrusion, in order to minimise risks. In water at a temperature of 20°C the film takes roughly 30s to degrade. Furthermore, the film can withstand a minimum mechanical compression of 300N (Newton) in normal dynamometric conditions, although some manufacturers have doubled this safety margin to up to 600N.
- 6.
A label has been placed on each capsule with precise hazard warnings, indicating the specific product type and brand, and the telephone number of the manufacturer. This is useful for parents and caregivers, but it has no positive effect for small children.16
- 7.
In addition to this, the material safety information sheets (HDSM) of various products will be changed because of the new raw material classifications.
The industry understands that new safety measures are launched gradually, and they will not be obvious immediately. They estimate that they will have a large impact 12–18 months after they are introduced into the market, as occurred in Italy after the package was made opaque after being alerted by the poison centres and confirming how the toxic exposures were reduced.17
It is important to ensure healthcare professionals receive this information (especially paediatricians), and to warn them about the greater risk of having LDCs in the home, in view of their greater toxicity compared to conventional laundry detergents.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: de la Oliva Urieta S, Mencías Rodríguez E, Ucha Domingo MS, Agudo Ordóñez J, Conejo Menor JL. Exposiciones tóxicas a las cápsulas de detergentes de ropa en España. Rev Esp Med Legal. 2016;42:17–23.