In recent months, much of the scientific efforts have focused on research on SARSCoV-2 infection and its consequences in humans. Still, many aspects remain unknown. It is known that the damage caused by SARS-CoV-2 is multifactorial and that its extension goes beyond lung inflammation and the acute phase, with the appearance of numerous complications and sequelae. To date, knowledge about the usefulness of 18F-FDG-PET/CT in the acute phase has been limited to the incidental detection of SARS-CoV-2 unsuspected pneumonia. Recent studies have been appearing collecting the findings of 18F-FDG-PET/CT in long COVID-19 or persistent COVID-19 state as well as the alterations caused after mass vaccination of the population in the metabolic studies. This work aims to review the existing literature focusing on these three issues and to briefly present our own preliminary experience.
Durante los últimos meses gran parte de los esfuerzos científicos se han centrado en la investigación sobre el SARS-CoV-2 y las consecuencias de su infección en humanos. Aun así, muchos aspectos siguen siendo desconocidos. Se sabe que la afectación por SARS-CoV-2 es multifactorial y que su extensión va más allá del daño pulmonar y del momento agudo, con aparición de numerosas de complicaciones y secuelas. El conocimiento de la utilidad de la 18F-FDG-PET/TC en el momento agudo se ha limitado, hasta la fecha, a la detección incidental de afectación pulmonar por SARS-CoV-2. En los últimos meses han ido apareciendo trabajos que recogen los hallazgos de la 18F-FDG-PET/TC en el estado post-COVID, asícomo las alteraciones provocadas en la imagen metabólica tras la vacunación masiva de la población. Este trabajo pretende revisar la literatura existente sobre estas tres cuestiones y exponer de manera breve la experiencia preliminar propia.
On December 31, 2019, the Municipal Commission of Health of Wuhan (Hubei, China) notified an outbreak of 27 cases of pneumonia. After confirmation of almost 32,000 cases, on February 11, 2020, the World Health Organization established the term Coronavirus Disease 2019 (COVID-19) to refer to this new disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). This new virus received this name due to the similarity with SARS in 2003, but it is recognized as a different entity. At the time of writing this work, the number of cases confirmed throughout the world was 169,597,415 (Spain 3,663,176) with a total of 3,530,582 deaths (Spain 79,888) and a global mortality of 2.1%, which may be as high as 37% among hospitalized patients1,2.
Information on COVID-19 has increased exponentially, as has the number of cases and almost 100,000 articles have been published in one year, the equivalent to the number of articles on the human immunodeficiency virus published over 14 years3. Nevertheless, many aspects of this new disease remain unknown.
PathogenesisThe physiopathology of SARS-CoV-2 infection is not completely deciphered. It is an encapsulated single-stranded RNA virus that belongs to the β genera of the Coronaviridae family together with MERS and SARS4. The main means of transmission is through liquid particles from the airway of infected persons. Once in contact with the host, SARS-CoV-2 penetrates into the cells by the binding of its Spike (S) glycoprotein to its principal receptor, angiotensin converting enzyme-2 (ACE-2). Encocytosis is facilitated by the transmembrane protein TMPRSS25. The ACE-2 receptors are strongly expressed in ciliated epithelial cells of the respiratory tract, in type II pneumocytes and in the alveolar macrophages and are also present in other tissues such as the brain, kidney, intestine, heart, uterus, suprarenal glands, gastrointestinal system, corneas and vascular walls. Internalization of the virus activates the immune system, producing the release of proinflammatory cytokines which, in turn, activate natural immunity (monocytes, T lymphocytes and macrophages) producing a feedback of the inflammatory response. Inadequate immune response produces an excessive accumulation of inflammatory cells and overproduction of cytokines and consequent tissue damage and extension of the process to different territories of the organism. The B lymphocytes which produce neutralizing antibodies also contribute to extension of the virus6. In addition to this exaggerated inflammatory and immune response, SARS-CoV-2 infection is also associated with coagulopathy, which is probably multifactorial in origin and involves endothelitis, endothelial dysfunction, alteration of blood flow and the activation of platelets and coagulation factors7. Lastly, the binding of SARS-CoV-2 to the ACE-2 receptor leads to an increase in circulating angiotensin-2 and a reduction of angiotensins 1–7, favoring a proinflammatory, profibrotic and vasoconstrictor state8.
Clinical manifestationsSince the airway is the main entry point for SARS-CoV-2, COVID-19 mainly presents with symptoms of infection of the respiratory tract, the upper tract in mild cases and pneumonia in severe cases. However, it is a multisystemic process in which other acute cardiac, thromboembolic, gastrointestinal and neurological complications, among others, may appear9–11.
The percentage of asymptomatic infected individuals ranges from 15 to 45%, and may be higher in children5. Among symptomatic patients, the mean incubation period is 5 days, with the appearance of symptoms beyond 11.5 days after exposure to the virus being infrequent. Most patients present mild symptoms (81%) with headache, cough, fever and/or slight dyspnea, but 14% of patients may develop severe clinical manifestations with greater dyspnea and hypoxemia, and 5% may develop a critical status with respiratory insufficiency or distress, shock and/or multiorgan failure5,12. The risk factors associated with a higher mortality are age greater than 65 years, active smoking, arterial hypertension, diabetes and cardiovascular or chronic pulmonary disease. With regard to analytical parameters, elevated C-reactive protein (CRP), lactate dehydrogenase, ferritin, procalcitonin, D-dimers and cardiac enzymes have also shown to be related to a worse prognosis3.
DiagnosisReal time reverse transcription polymerase chain reaction (RT-PCR) of respiratory samples is considered the diagnostic reference standard with a specificity of almost 100%. However, its sensitivity ranges between 60–97% due to failure of adequate sample collection, performance in different stages of the disease or due to a low viral load3.
Simple radiography and chest computerized tomography (CT) play a fundamental role in the diagnosis of SARS-CoV-2 pneumonia and its complications. Radiography may be normal in the initial phases or in mild forms of the disease4,13. CT is more sensitive and can demonstrate alterations in up to 50–70% of asymptomatic individuals13. The most common findings are ground glass opacities in the lungs (50–98%) or a reticular radiological pattern (in up to 77% of the cases) with a bilateral distribution of peripheral and posterior predominance. Consolidations alone or overlapping the ground glass opacities may also be present (24.2–64%). Other findings such as a cobblestone pattern, pleural effusion or mediastinal adenopathies have been observed in a minority of cases14. The frequency of the findings depends on the time at which the exploration is made during the course of the disease. Ground glass opacities generally develop between days 0–4 after the onset of symptoms and achieve a maximum at 6–13 days. According to the course of the disease, the frequency of consolidation increases15. A recent meta-analysis demonstrated that determined findings are related to greater severity of the process, such as hilar mediastinal adenopathies, traction bronchiectasis, interlobar septal thickening, consolidations, cobblestone pattern, reticulation or pleural effusion16.
Despite the elevated sensitivity of the radiological tests, in April 2020, the Fleischner Society declared an international consensus which, considering the limitation of resources, established that imaging studies are not indicated in asymptomatic patients or in those with mild symptoms because of the absence of the risk of worsening. This consensus also did not recommend serial studies in stable intubated patients. The indication of imaging studies was considered in patients with moderate or severe clinical manifestations independently of the PCR result or in patients diagnosed with COVID-19 with respiratory worsening or functional deterioration and/or hypoxemia following COVID-1917.
TreatmentGiven the complexity of the physiopathology of COVID-19 and its clinical versatility, there is no standard treatment, and the response to therapy is variable.
Antiviral drugs and plasma antibodies of convalescent patients or hyperimmune immunoglobulins have been used in the treatment of SARS-CoV-2. Anti-inflammatory, immunomodulator, anticoagulant and antifibrotic treatments have been used in relation to host response12.
More than 75% of hospitalized patients require supplementary oxygen support which may vary from nasal oxygenotherapy to invasive ventilation in severe cases. Dexamethasone has been associated with a reduction in the risk of mortality in patients with severe COVID-19, and its use is recommended in patients requiring oxygen (IA evidence), with no demonstrated benefits in patients in whom oxygen therapy is not indicated. Tocilizumab in combination with dexamethasone reduces the risk of progression to invasive mechanical ventilation and mortality18.
In the hospital setting wide spectrum antibiotherapy is often associated to cover possible fungal or bacterial infections.
Persistent symptoms or post-covid-19 statusIncreasingly more clinical and scientific evidence has been obtained regarding the subacute and long-term effects of COVID-19. Nonetheless, there is still no universally accepted definition, and these symptoms have not been classified as a disease or a clear syndrome. However, it has been proposed to differentiate at least 3 times of the disease19:
- -
acute COVID-19: symptoms and signs caused by SARS-CoV-2 which may last up to 4 weeks after the onset of the clinical manifestations.
- -
Sequelae of COVID-19: sequelae produced after acute SARS-CoV involvement which frequently occur in patients who required hospitalization, sometimes in the intensive care unit (ICU). These are the consequence of structural damage to different organs by the infection itself and/or associated complications. They include sequelae of the critical state itself and the possibility of secondary infections.
- -
persistent or “Long COVID”: symptomatology which persists 4–12 weeks after acute infection in patients diagnosed with COVID-19 in whom confirmation of COVID-19 by laboratory tests is not mandatory. Some authors have proposed calling this process subacute COVID to differentiate it from chronic or post-COVID syndrome COVID, which would involve symptoms which persist or appear beyond week 1220.
The pathogenesis of the post-COVID state remains unknown, but it seems that the persistence of a state of inflammation plays a fundamental role, with these patients presenting elevated CRP and interleukin-6 levels in blood. It is likely that other factors such as persistence of the virus in tissues causing latent or chronic infections, the possibility of reinfection, low ACE-2 receptor regulation and vascular dysfunction also contribute. Other factors that have been proposed include physical deconditioning due to prolonged inactivity, neuropsychological factors due to post-traumatic stress and dysregulation of the sympathetic nervous systems19–21.
Persistent COVID has been described in up to 87% of patients requiring hospitalization in the acute phase of the disease, with the presence of at least 3 symptoms in 55% of the cases20. The clinical spectrum is very wide, and it is not accurately known why some persons take longer to recover. It can occur in not only patients who have presented an acute infection with a severe course but also in those who have had a milder form of the disease, and while it can affect people of any age or sex, it predominates in women between 36–50 years of age without previous comorbidities22. In general, the symptoms fluctuate and are cyclic, alternating days in which they have practically disappeared with others in which they reappear. The most frequent symptom is fatigue, presented in 17–72% of the patients who have been hospitalized, followed by dyspnea, and joint, muscle and chest pain. However, many other symptoms may appear and affect practically any organ, with other sequelae or pulmonary (cough and reduction in diffusing capacity for carbon monoxide), cardiovascular (palpitations, arrhythmias, tachycardia, disautonomy and myocardial fibrosis), hematologic (thromboembolic phenomenon) neuropsychiatric (headache, disautonomy, parestesia, brain fog, anxiety, depression, sleep disorder, anosmia and dysgeusia), renal (reduction in glomerular filtration), endocrinological (poor control of diabetes, subacute thyroiditis, hypothyroididm, bone demineralization), digestive (diarrhea and abdominal pain, alteration of the intestinal microbiota), dermatological complications, pediatric multisystemic inflammatory syndrome and fever or low-grade fever20,21,23–25. This symptomatology has been described in other coronaviruses (SARS, MERS) and in infection by the Ebstein–Barr virus and chikungunya, among others, resolving after several weeks or months.
Studies able to demonstrate this state are essential to plan treatments and the care of these patients and direct the focus of future lines of investigation. While there is still no evidence on the efficacy of pharmacological or non pharmacological measures versus this persistent symptomatology, multidisciplinary management is recommended22,26.
F-FDG PET/CT in SARS-CoV-2 infectionRespiratory systemAs mentioned previously, viral pneumonia activates the inflammatory cells (neutrophils, monocytes and T-helper lymphocytes) and the local release of cytokines27. Considering the avidity of inflammatory cells for glucose, it is to be expected that the disease can be evaluated by the fluorodeoxyglucose F 18 positron emission tomography/CT (18F-FDG-PET/CT) technique that has shown to be useful in the study of infectious and inflammatory diseases in general and especially in the respiratory tract28. Previous studies have demonstrated that patients infected by MERS showed marked pulmonary uptake of 18F-FDG in progression to pneumonia29. Likewise, pathological uptake of 18F-FDG has been described in mediastinal and axillary adenopathies in non human primates 5 days after having been infected by MERS, coinciding with monocytosis in peripheral blood with no changes in the CT images or in body temperature30. Studies performed in camels and rabbits infected with MERS showed the presence of virus in the absence of clinical signs, speculating that subclinical infection could also occur in healthy immunocompetent humans, since the majority of patients presenting severe lower respiratory tract infection had underlying comorbidities31. 18F-FDG-PET/CT has also demonstrated activation of the lymphoid tissue of axillary, cervical and mediastinal lymph nodes, which precedes fulminant replication of the virus, in macacos infected with the simian-human acute immunodeficiency virus32.
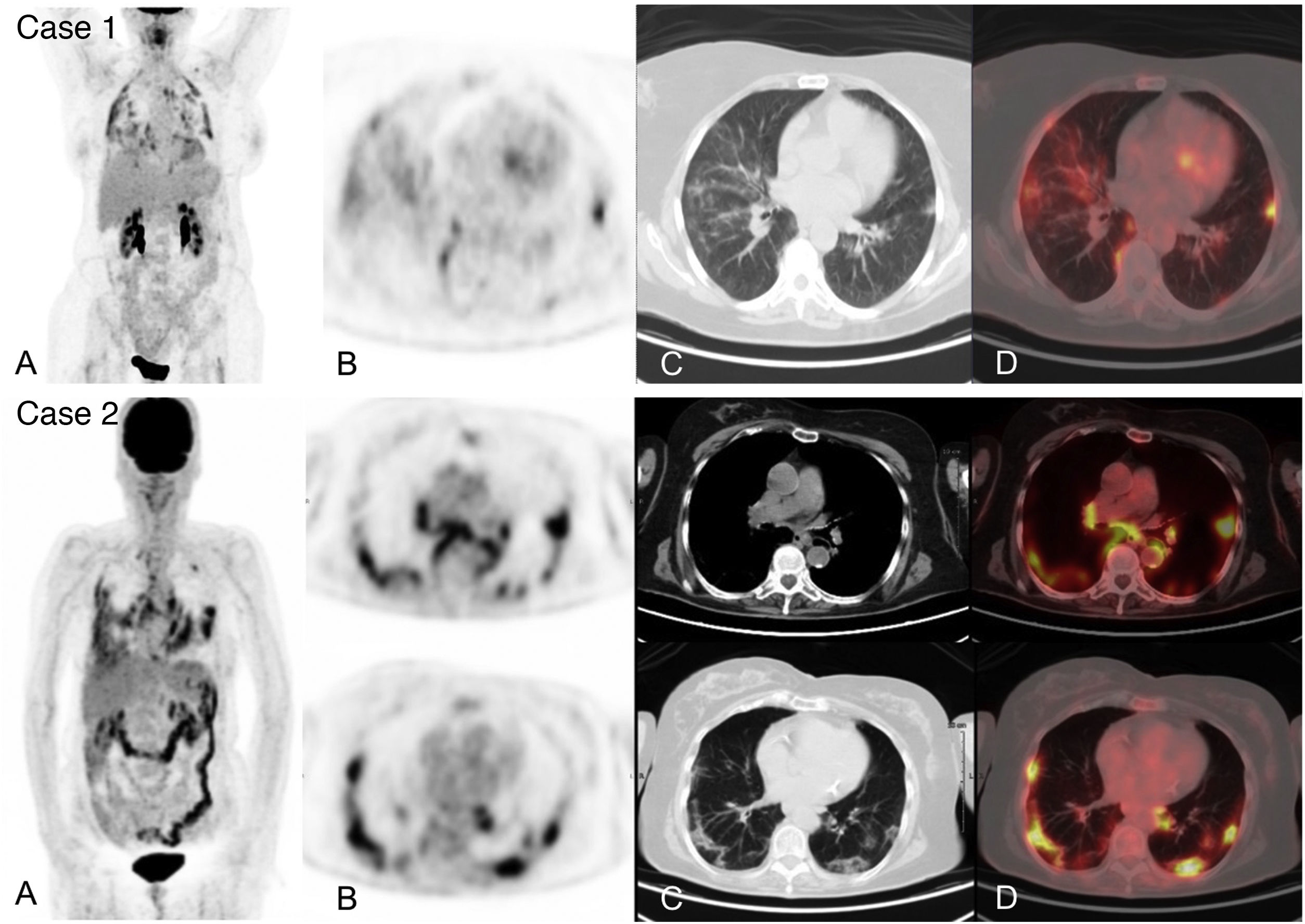
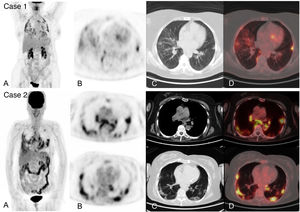
18F-FDG-PET/CT can demonstrate characteristic and early radiological alterations of COVID-19 pneumonia despite using low-dose CT which, however, is also diagnostic33. Numerous studies have described incidental detection of metabolic alterations in the pulmonary parenchyma of often asymptomatic patients undergoing a 18F-FDG-PET/CT for any established clinical indication, in whom SARS-CoV-2 infection was later confirmed, with a frequency ranging from 4.1 to 9.2%34–37 (Fig. 1. Case 1). The appearance of these alterations in asymptomatic patients may reflect early immune response to the infection similar to what was reported in the previously mentioned animal models.
Acute infection by SARS-CoV-2 detected during an 18F-FDG-PET/CT study in 2 asymptomatic patients from a respiratory and infectious point of view, later confirmed by RT-PCR. Case 1. MIP (A) of a patient with diffuse large cell B lymphoma in complete metabolic response showing multiple pulmonary uptakes also visible in the axial PET slice (B) and corresponding to ground glass opacities in the axial CT (C) and PET/CT slices (D).
Case 2. MIP (A) of patient followed for melanoma showing intense pulmonary and hilar mediastinal uptake corresponding to bilateral ground glass opacities of peripheral predominance and mediastinal and hilar adenopathies visible in the PET (B), CT (C) and PET/CT tomographic slices (D).
A recent systematic review described the findings observed in 52 patients with COVID-19 pneumonia incidentally detected following a PET/CT study with 18F-FDG in 48 cases, 18F-choline in 3 and with 68Ga-PSMA in 1 case. The most frequent findings were hypermetabolic bilateral ground glass opacities (75%), consolidations (34.6%), and interlobar septal thickening (7.6%). The range of the maximum standard uptake value (SUVmax) observed in studies with 18F-FDG was 2.2–18 (mean SUVmax 4.9 ± 2.3), between 3–4 in cases with 18F-choline, and in the only case with 68Ga-PSMA, the SUVmax was 3.238. It has been observed that pulmonary lesions with greater 18F-FDG uptake could be correlated with a greater globular sedimentation rate and with a greater time to curation, although cohort studies in a larger number of patients are needed to draw robust conclusions in this regard39,40.
Perhaps the most distinctive finding of the 18F-FDG-PET/CT studies performed in patients with COVID-19 pneumonia is the presence of mediastinal (27%) and hilar adenopathies (19.2%) with pathological uptake, which were infrequent in radiological studies published to date16,38 (Fig. 1. Case 2).
Despite the capacity of the technique to demonstrate pulmonary disease, in general, 18F-FDG-PET/CT is not recommended as a diagnostic procedure in COVID-19 pneumonia since it seems to provide little over chest radiography and CT14. In addition, although 18F-FDG-PET/CT reflects the inflammatory pulmonary process in the supposed peak of the acute phase of COVID-19, no correlation has been demonstrated between inflammatory status and the evolution of the radiological findings or short-term clinical outcomes41. It should also be taken into account that this type of study increases the exposure time of professionals and other patients to the virus compared to CT or radiography which are more rapid techniques.
The “normal” period of resolution of the pulmonary alterations in patients who have had pneumonia is not exactly known. The cohort with the longest follow-up after hospital discharge included 1733 patients, 353 of whom underwent CT study, finding persistent radiological alterations in approximately 50% of the cases, which, overall, were consistent with ground glass opacities.
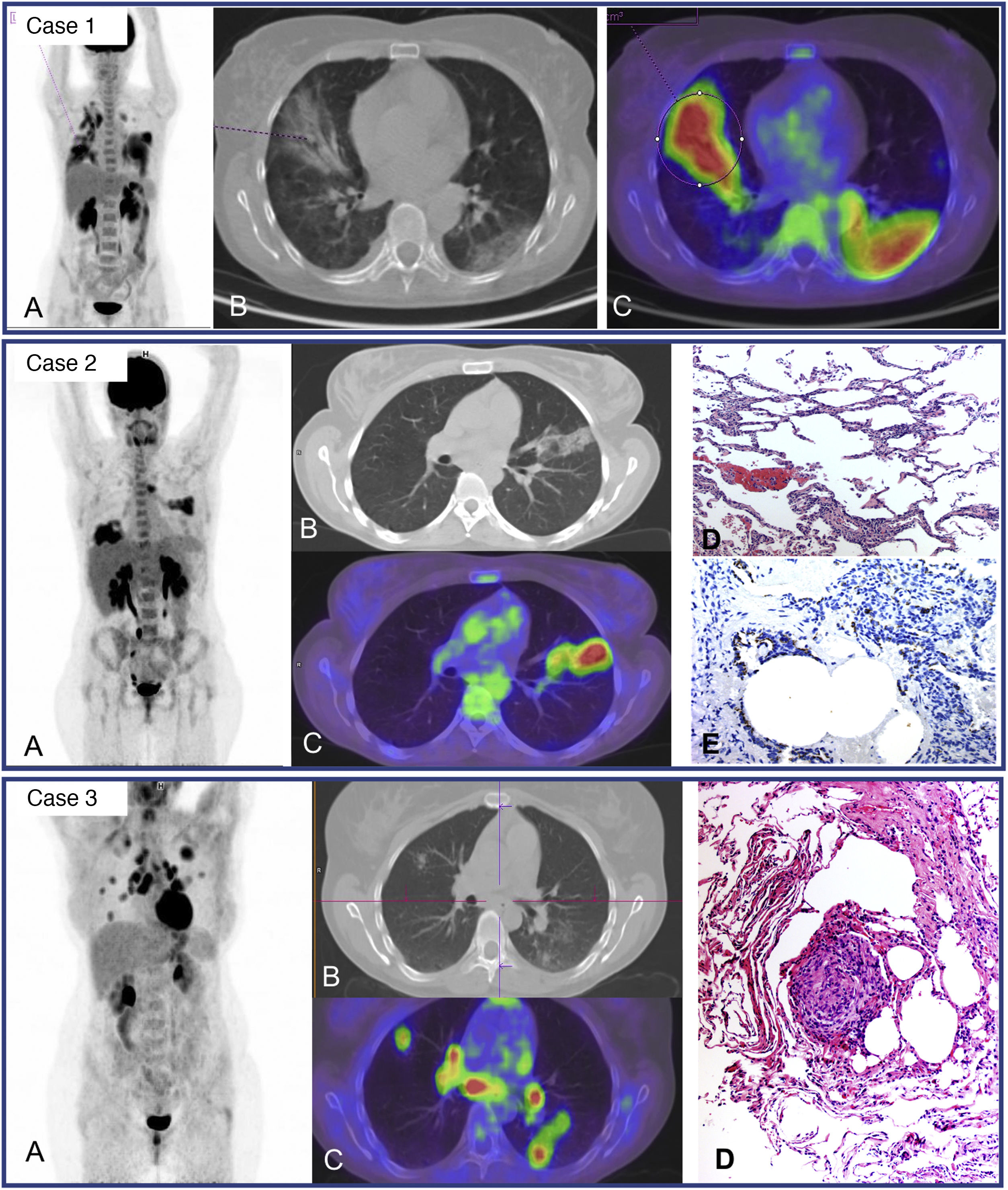
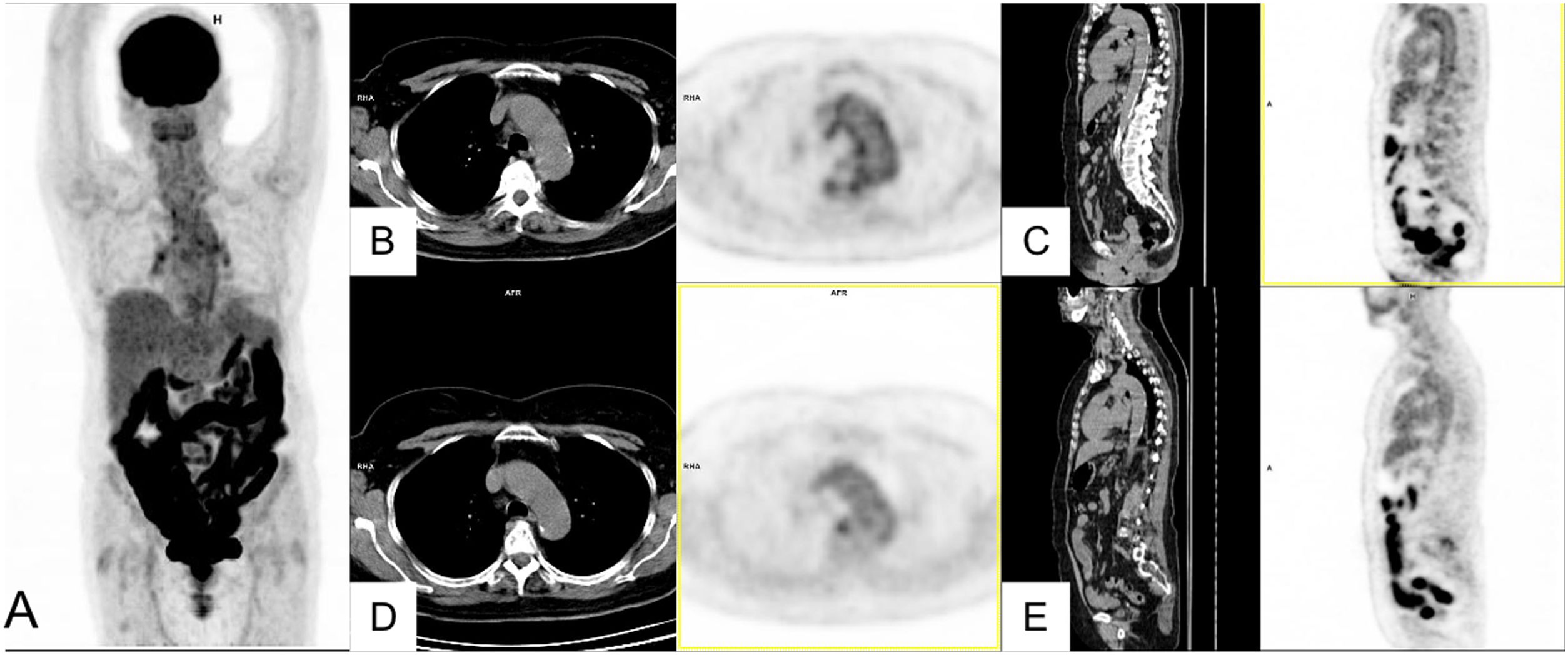
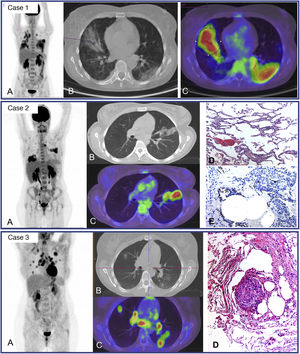
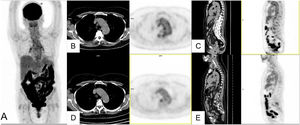
The uptake described in residual pulmonary lesions in patients recovering from COVID-19 pneumonia between the 2nd and 4th week after the establishment of symptoms was mild-moderate (SUVmax 3.4 ± 2.3) and was even lower after the first month (SUVmax 0.85–1.79)42,43. Since the beginning of the pandemic we have studied 24 patients for persistence or worsening of systemic COVID-19 symptoms, with a time from symptom appearance or since hospital admission of at least one month. We detected elevated-moderate intensity of pulmonary uptake in 8 patients, coinciding with areas of parenchymatous alterations in CT consisting in ground glass infiltrates with different grades of consolidation. Six patients shared the common characteristic of having received immunosuppressive treatment for previous diseases and presented fever or persistently elevated low fever. Bronchoalveolar lavage performed after the PET/CT showed an elevated percentage of macrophages (45–81%) and a variable percentage of lymphocytes (4–51%). In one patient, the presence of SARS-CoV-2 was demonstrated by PCR of the bronchoaspirate, despite repeatedly negative nasopharyngeal samples (Fig. 2. Case 1). In 3 other patients, immunohistochemistry of pulmonary tissue was performed in 2, showing positivity versus the S2 subunit of the surface Spike protein of SARS-CoV-2 (Fig. 2. Case 2). In the remaining cases immunohistochemistry was not performed since they occurred prior to the availability of the technique. These results warn of the possibility of the virus remaining beyond the acute phase in immunosuppressed patients.
Case 1. MIP (A) of patient with fever of unknown origin and Sjögren syndrome receiving treatment with rituximab. Clinical picture compatible with COVID-19 (fever and CT with bilateral parenchymatous involvement) since March 25, 2020 with 3 PCR-negative nasopharyngeal exudates. PET/CT 1 month later showing intense bilateral pulmonary uptake coinciding with radiological alterations visible in the axial CT (B) and PET/CT slices (C) and probable medullary reactivation. A posterior bronchoaspirate was performed confirming persistent infection by SARS-CoV-2 in the PCR.
Case 2. MIP (A) of patient with multiple sclerosis receiving treatment with rituximab and post-COVID fever of 2 months of evolution. Pathological uptake of elevated intensity is observed in both pulmonary parenchymas coinciding with areas of opacification in the CT and PET/CT (B,C). A transbronchial biopsy was later performed showing T lymphocyte pneumonitis (D), and immunohistochemical staining showed the persistence of SARS-CoV-2 in lung tissue. (E).
Case 3. MIP (A) of young patient with no history of interest with post-COVID dsypnea of 8 months of evolution. Multiple nodular uptakes are shown in both lungs and bilateral hilar mediastinal uptake, coinciding with areas of opacification of the parenchyma and adenopathies which were not increased in size (B, C). The histological lymph node study obtained by echobronchoscopy (D) showed non necrotizing sarcoid type granulomatous inflammation. The posterior transbronchial biopsy showed the same type of granulomatous inflammation without the presence of virus in the immunostaining.
The other 2 patients with moderate-elevated pulmonary uptake did not have a history of immunosuppression. One case in which PET/CT was requested due to persistent high fever corresponded to bacterial superinfection, showing clearly dominant uptake in the left lung. In the second case, the PET/CT was carried out due to persistent low fever and significant dyspnea discordant with the radiological involvement. The study showed intense pulmonary uptake coinciding with nodular opacities of variable size as well as multiple hilar mediastinal lymph nodes which were not increased in size. Fine needle aspiration puncture of the subcarinal adenopathy was performed showing a sarcoid reaction for which high dose corticoid treatment was initiated (Fig. 3, Case 3).
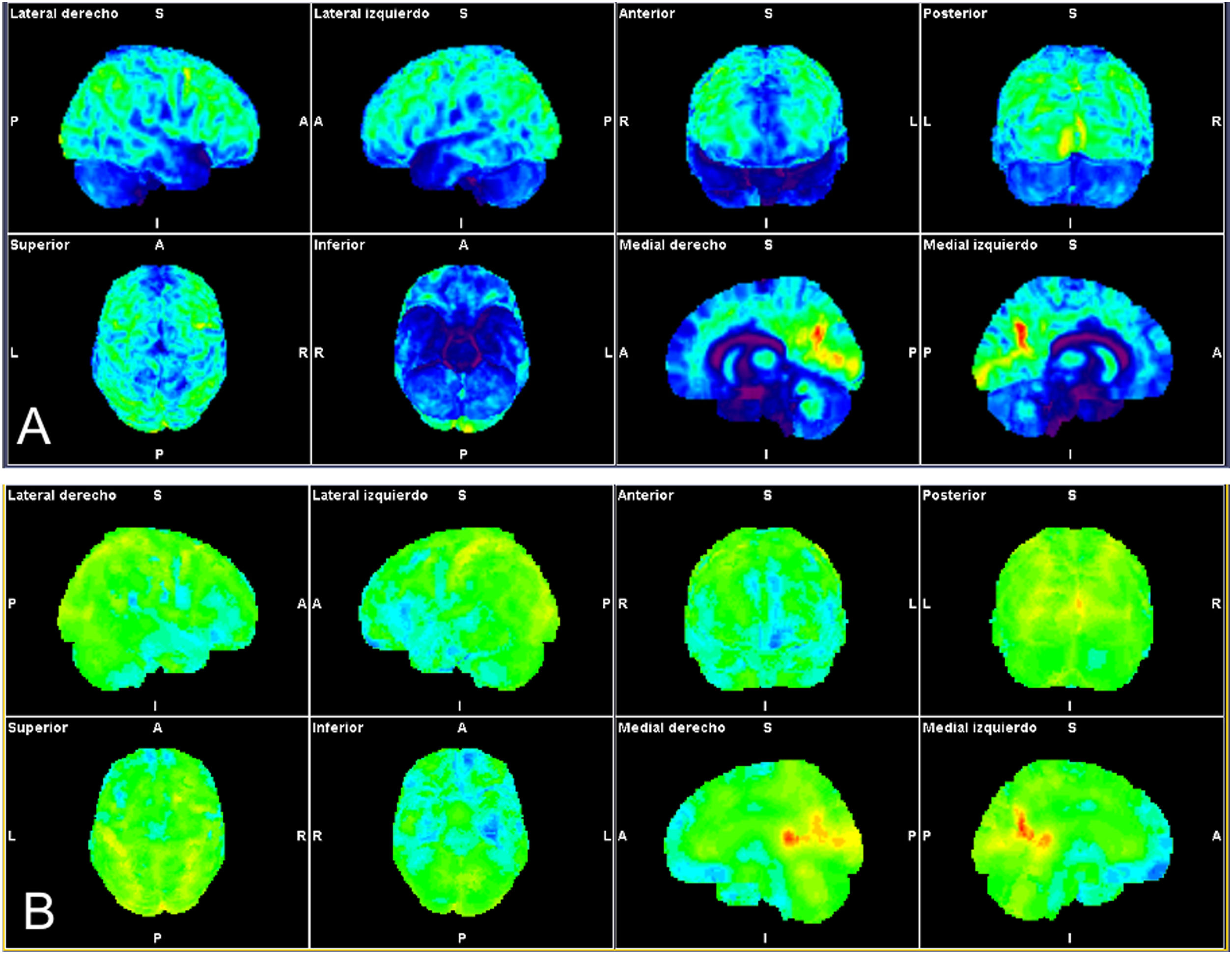
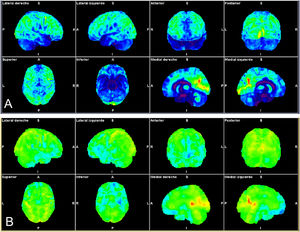
Patient with autoimmune profile (history of sacroileitis, uveítis, sensitivity to gluten) and mild SARS-CoV-2 infection with progressive loss of short-term memory, attention deficit and important asthenia. The statistical brain surface maps obtained by automated analysis are shown (Syngo.via Neurology; Siemens). CT images showing normalization of the whole brain (A) and comparative analysis with the database adjusted for the age of the patient (B) showing frontal hypometabolism (fronto-orbital, rectal and inferior gyri) bilateral temporal bilateral (amygdalas and hippocampos), in both thalamuses and brain stem relative to both cerebellar hemispheres.
Based on our experience, within the framework of investigation of post-COVID status, elevated pulmonary uptake beyond the first month of the acute process with no evident cause constitutes a sign of alarm and justifies targeted studies.
One of the most concerning sequelae is pulmonary fibrosis. According to a recent meta-analysis, approximately 30% of patients hospitalized for SARS-CoV-2 pneumonia demonstrate fibrotic changes which persist the first 12 months after discharge44. Given the time since the initiation of the pandemic, it is not known if this fibrosis will remain stable or whether, as with idiopathic pulmonary fibrosis (IPF) with which it shares common pathogenic pathways, it will have a progressive nature45. In IPF, 18F-FDG PET/CT studies have shown a significant inverse association between the level of pulmonary uptake and survival, independently of age, sex and physiological variables, being maintained even in patients with good pulmonary function. Therefore, if the irreversible and progressive post-COVID nature of fibrosis is demonstrated, PET/CT could be useful as a prognostic biomarker and contribute to designing treatments46.
Cardiovascular systemCardiovascular comorbidity has been associated with greater severity of COVID-19, with evidence of cardiac lesions in 7–28% of hospitalized patients, showing a substantially higher mortality rate47,48. In addition, there is increasingly greater evidence that acute respiratory disease is significantly associated with acute myocardial damage, which appears in up to 20–30% of patients49, the mechanism of which has yet to be clarified. Autopsy series of patients with COVID-19 have found viral particles in the myocardium in 47% of cases, and more than 7.5% of the myocardial cells express ACE-250,51. It is likely that vascular and myocardial hyperinflammation mediated by cytokines, endothelial dysfunction, hypercoagulability and the effects of systemic infection on the heart are involved in this damage51.
In patients with a significant elevation of biomarkers of myocardial damage, imaging studies may be useful to clarify the origin, although the indication during the acute phase of the infection should be carefully weighed given the risk of exposure to the virus by personnel and patients. In hemodynamically stable patients with known coronary disease and chest pain of uncertain origin and acute coronary syndrome without ST elevation, perfusion SPECT or PET/CT studies with pharmacological stress are useful, but if there is low risk the study can be deferred until resolution of the acute phase52.
Patients hospitalized for COVID-19 have a greater risk of associated fungal or bacterial infection, including endocarditis, in which 18F-FDG-PET/CT has shown an impact in 40% of the patients53. This indication should, therefore, be taken into account even in patients with active infection.
The prevalence of myocarditis associated with COVID-19 is unknown. A systematic review of autopsy studies has reported a prevalence of 1.5% of the cases50, although other studies refer up to 7% of fulminant myocarditis in the acute phase of the disease. Follow-up studies of patients hospitalized for COVID-19 describe the presence of alterations in magnetic resonance (MR) studies in 75% of the cases, with a diagnosis of pericarditis in 3% of the patients, myopericarditis in 11% and myocarditis in 26%, being asymptomatic on many occasions54. The detection of persistent inflammation is important since it may lead to the development of dilated myocardiopathy and potentially fatal arrhythmias. Delayed enhancement of gadolinium in MR, which is a sign of active inflammation in acute phases, loses specificity in the advanced phase due to greater difficulty in differentiating active inflammation from scarring or fibrosis. 18F-FDG-PET/CT provides additional information to the differential diagnosis, with a specificity of 97%49,55 by demonstrating uptake in the lesions corresponding to inflammation. To date, no study has analyzed the utility of 18F-FDG-PET/CT in myocarditis by SARS-CoV-2, but it has already shown to be useful in the evaluation of ventricular dysfunction when other inflammatory myocardiopathies are suspected56. The detection of myocardial inflammation and its response to corticoid treatment by 18F-FDG-PET/CT have been described in a pediatric patients with multisystemic inflammatory syndrome after infection by SARS-CoV-257.
Hopefully, the recently initiated CardiOvaScular Mechanisms In Covid-19 (COSMIC-19) study will clarify the presence and characteristics of cardiac lesions associated with SARS-CoV-2 infection and the utility of 18F-FDG-PET/CT for its characterization58. For now, and until more information is available, it may of interest to apply a protocol of preparation of myocardial suppression59 in 18F-FDG-PET/CT studies performed within the context of post-COVID-19 status taking into account the description of palpitations and chest pain 6 months after discharge in 9% and 5%, respectively of patients hospitalized for COVID-1924 and the reported presence of alterations in MR even in asymptomatic patients.
Embolic phenomena have been described in up to 5% of patients after acute infection. The duration of the prothrombotic state is unknown. Preliminary studies indicated less than 5% of thromboembolic events after acute COVID-19. The predisposing risk factors identified include a elevation of D-dimer 2-fold above the normal values, intercurrent neoplasia, a body mass index greater than 30, age over 60 years, recent admission to an ICU or a previous thromboembolic episode20. Some of these characteristics are present in patients referred for PET/CT study, and thus, we should be especially thorough in the review of pulmonary vascularization, especially in centers using a CT acquisition protocol with intravenous contrast.
Nervous systemIt is suspected that SARS-CoV-2 has neuroinvasive potential and viral particles have been demonstrated in endothelial cells of the frontal cortex in histological studies60. In addition, ACE-2 receptors are expressed in vascular endothelium and in nerve cells in both neurons and glial cells, especially in the ventrolateral territory of the medulla, mesenchaphic black matter, medium temporal gyrus, posterior cingulate cortex and the olfactory bulb61,62. Several mechanisms of entry of the virus into the central nervous system have been considered, such as transynaptic retrograde dissemination from the olfactory bulb, direct vascular invasion by adhesion to endothelial cells passing the hematoencephalic barrier or as a Trojan horse in the interior of infected macrophages and lymphocytes63. Apart from the direct damage caused by the virus, there are mechanisms of indirect damage mediated by cerebral hypoxia, activation of the microglia secondary to elevation of cytokine levels by the blockade of ionic receptors or canals by antibodies by activation of the hypothalamic-hypophyseal axis and by vascular and thrombotic phenomena64 (Fig. 3A). Post-mortem series analyzing brain tissue have found the presence of the virus in 53% of the cases, with no relationship with the severity of neuropathological changes. The dominant findings were the presence of astrogliosis, activated microglia and cytotoxic T lymphocytes in the frontal cortex, cerebellum and basal lymph nodes as well as the presence of multiple areas of infarction65.
In the acute phase of the disease the majority of clinical series describe the appearance of neurological symptoms mainly in patients hospitalized due to the severity of the respiratory condition62,66. According to the largest series66, up to 57.4% of the patients present unspecific neurological symptoms such as headache, reduction in the level of consciousness, agitation, alterations in smell and/or taste, myalgias and more specific conditions, such as cerebral infarction, encephalitis and white matter lesions in imaging studies67.
Although there is a lack of specific biomarkers of cognitive deterioration produced by SARS-CoV-2, some studies have shown that the inflammatory phenomenon related to infection could preferentially be directed at the frontal lobes and/or frontal neuronal networks in that behavioral and dysexecutive symptoms predominate, as well as frontotemporal hypoperfusion in MR, and deceleration of the electroencephalogram in the frontal regions and frontal hypometabolism in 18F-FDG-PET/CT studies is observed in not only patients with frontal syndrome but also in those without delirium but with anosmia or ageusia68. Cases pf acute encephalopathy by COVID-19, encephalitis by SARS-CoV-2 or sensitive to steroids have frequently been reported in hospitalized patients with acute delirium and other neuropsychiatric symptoms. Early 18F-FDG-PET/CT studies in these patients show prefrontal or orbitofrontal hypometabolism of the anterior insular and cingulate cortex and in cerebellar, and less frequently, in caudate hemispheres. In the acute phase, hypermetabolism in the vermis and striates may also appear and is attributed to compensatory, electroconvulsive or inflammatory phenomena69,70. Sequential PET/CT studies performed 1 and 6 months after the basal image show the disappearance of hypermetabolic areas and less hypometabolism consistent with clinical improvement. This indicates that the metabolic alterations may be the neuronal substrate of the clinical manifestations of the patients which follow, overall, with a frontal syndrome, mood disorders and alteration of the perception of respiratory failure70.
18F-FDG-PET/CT has also been used to study 2 patients with acute COVID-19 and cognitive-motor dissociation or covert consciousness, a condition which occurs in hospitalized patients due to severe brain lesions and who do not seem to respond to orders but preserve their cognitive capacity in contrast to real disorders of consciousness. Diffuse frontal hypometabolism with respect to the motor and premotor cortex has been observed but affecting associative areas responsible for the integration of motor initiation and coordination. These results demonstrate the utility of 18F-FDG-PET/CT in patients with severe COVID-19, normal MR, isolated motor deficit in the electromyography and absence of response, confirming the integrity of the structures responsible for voluntary movement71.
A case of acute anosmia with mild hypometabolism in the left orbitofrontal cortex has been published suggesting neuronal deterioration due to the direct action of SARS-CoV-272. However, a more recent study suggests that anosmia seems to be related to deaferentiation and functional reorganization processes due to a lack of olfactory stimulus leading to subtle metabolic changes in olfactory and associative cortical areas visible in 18F-FDG -PET/MR73.
There is increasing evidence of the persistence of neurological symptoms following acute COVID-19, a process recently denominated Neuro-PACS (neurological manifestations of post-acute sequelae of SARS-CoV-2 infection)74. The incidence of this process is greater in patients requiring hospitalization, especially in the ICU or in those who develop encephalopathy. This symptomatology varies greatly. According to an electronic registry of the follow-up of 236,379 patients during the first 6 months after the diagnosis of COVID-19, neuoropsychiatric sequelae appeared in 34% of the cases, without including headache. The most common sequelae include mood disorders and anxiety and psychosis (24%), neuropathies (2.1%), ischemic infarctions (2.8%) and dementia (0.67%)75. The possibility of developing Parkinsonism after COVID-19 is also of concern, considering the sequelae of previous pandemics such as the 1918 influenza pandemic. Involvement of the nigrostriatal system after infection by SARS-CoV-2 has been described in neuroimaging studies76. However, it not clear whether the risk of Parkinsonism is greater than that after other infections of the respiratory tract75.
The most frequent symptoms in our setting are headache and cognitive complaints described in up to 68% and 81%, respectively, of patients with any neurological symptom beyond week 12 after the acute infection77. The cognitive complaint usually reported is a lack of concentration and lack attention or difficulty in task planning, which have been joined under the term of brain fog, although the neurocognitive profile has not yet been correctly described.
18F-FDG PET/CT studies performed in hospitalized patients with at least two neurological symptoms in the subacute phase of the disease show a reduction in metabolism with frontoparietal predominance correlated with alteration of the Montreal Cognitive Assessment with a profile of neocortical dysfunction78.
It has been hypothesized that persistent functional complaints (fatigue, dyspnea, alteration of taste and/or smell, memory or cognitive decline, sleep disorder and pain, among others) after acute infection by SARS-CoV-2 may be related to central involvement which may be manifested by 18F-FDG PET/CT using this technique as a diagnostic biomarker and for follow-up. In this sense, retrospective analysis of brain metabolism in 35 patients with this symptomatology, compared with the control group, showed hypometabolism which fundamentally affected the orbital and rectal gyri of the right temporal lobe, with extension to the ipsilateral thalamus of the protuberance and the cerebellum. In addition, these hypometabolic areas were related to determined symptoms. Patients with a greater number of complaints showed greater involvement of the brain stem and the cerebellum. The greatest frontal hypometabolism was detected in patients with pain. Cognitive complaints were related to greater involvement of the cerebellar hemispheres79.
Another study in patients with persistent symptomatology beyond one month after the primary infection found a relationship between hyposmia/anosmic and a characteristic hypometabolism of the parahippocampal gyrus and bilateral orbitofrontal cortex and between fatigue and the right parahippocampal gyrus, the brain stem and the bilateral thalamus80.
According to our preliminary experience pending validation in 10 patients with brain fog of new appearance following COVID-19 presenting attention and dysexecutive deficit referred from the neuroCOVID consultation, metabolic involvement in the 18F-FDG-PET/CT showed mainly left inferior frontal predominance of the temporal territory also with left predominance, and of both cerebellar hemispheres. Similar to the study of Guedj et al.79, the patients with the greatest systemic functional involvement demonstrated greater alterations in the brain PET/CT study (Fig. 3).
Astrocytes significantly contribute to cerebral uptake of 18F-FDG, and they are known to play a main role in defence against inflammatory aggression. Although the cellular substrate of the persistent cerebral hypometabolism with 18F-FDG-PET/CT has not yet been defined, the persistence of astrocytary alteration identified in post-mortem studies could be a main cause81.
PET in post-COVID-19 stateThe tools for the diagnosis of the post-COVID state have yet to be established and possibly depend on the dominant symptoms. Considering their physiopathology, a technique such as 18F-FDG-PET/CT may offer advantages in these patients by its ability to demonstrate areas of inflammation and/or hypofunction as well as foci of active infection and provide a global evaluation of the organism. In addition, the sensitivity of this technique can be increased since the affinity of the glucose transporter receptors to 18F-FDG increases the presence of cytokines and growth factors80. Nonetheless, we must be cautious since in daily practice it would be impossible to assume the total demand generated by patients in the post-COVID state. Therefore, it is necessary to evaluate the syndrome more in depth, maintaining fluid communication with the departments involved to establish a clinical framework of greater utility or diagnostic performance.
In this article we have revised the utility or contribution of 18F-FDG-PET/CT in the complications or specific sequelae of COVID-19, but, to date, only one study has reported systemic findings of 18F-FDG-PET/CT images in patients with unspecific symptoms beyond the first month, including dyspnea (69%), fatigue (62%), ageusia/anosmia (31%), joint pain (23%) and tachycardia (15%), among others. The results indicate that compared with the control group, in these patients there is greater multiorgan uptake, especially in the bone marrow, as well as vascular and in large joints, suggesting a state of persistent systemic inflammation, without being able to define a concrete metabolic pattern.
In our setting, referral for a 18F-FDG-PET/CT study is mainly made due to persistent low fever/fever or significant constitutional syndrome with or without the remaining symptoms described. The objective of the 18F-FDG-PET/CT study is, overall, to rule out the presence of concomitant pathological processes.
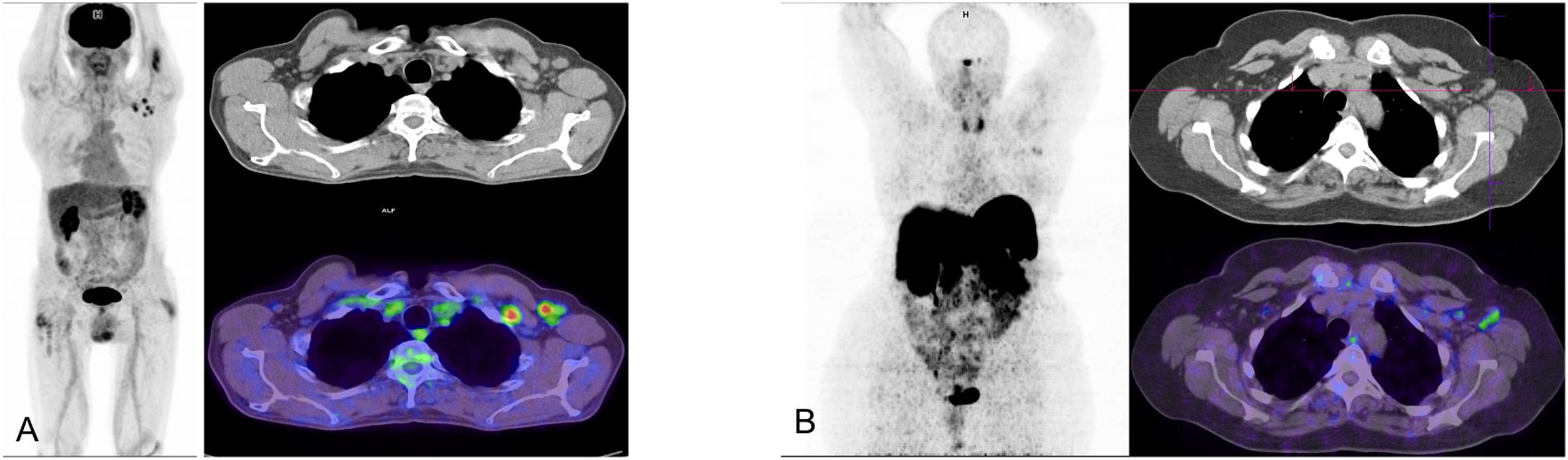
Of the 24 studies carried out, 8 showed significant pulmonary uptake (33%). In the remaining studies no reproducible pattern was found, except for diffuse uptake in the major salivary glands in 6 cases (25%) together with unspecific muscle uptake in one and thymic in another. Five patients (21%) showed physiological distribution of the radiotracer with no notable pathological findings. The fact of not having detected the multiorgan increase described by Sollini et al.80 is likely due to a greater time between acute infection and the imaging study. In 2 cases hypermetabolic hilar mediastinal adenopathies were detected and histologically confirmed as sarcoid reaction in one case (Fig. 2. Case 3) and not investigated in the other. One papilar thyroid carcinoma was incidentally identified as well as 1 case of diffuse increased uptake corresponding to hypothyroidism. A prevalence of 5.2–5.6% of hypothyroidism has been reported in COVID-19 patients, but it remains unknown whether hypothyroidism was previously present in these cases or if it is a secondary phenomenon to the activation of the autoimmune process by the infection82. Lastly, one patient showed diffuse pathological uptake in the wall of the thoracic aorta. In this case, vasculitis was clinically suspected, with an initial study with 18F-FDG-PET/CT showing no findings of interest. Following COVID-19, the clinical manifestations worsened in consonance with the demonstration of pathological vascular uptake (Fig. 4). The possibility that the infection had acted as a phenomenon of activation of a previous autoimmune process was considered.
Patient with general malaise and weight loss and elevation in C-reactive protein following mild COVID-19. The patient was followed for a polymyalgic picture as the presentation of HLAB27+ enthesopathy. MIP (A) showing a diffuse increase of intestinal uptake attributed to oral antidiabetic treatment and slight bilateral hilar uptake and of the thoracic aorta. Axial (B) and sagittal slices (C) of CT and PET showing an increase of uptake in the aorta wall, not visible in a previous study performed for a rhematological picture (D, E).
The main contribution of 18F-FDG-PET/CT in our patients post-COVID-19 with an unfavorable evolution has been to confirm or rule out other significant pathological processes that could justify the clinical manifestations.
Changes in PET/CT imaging induced by vaccination against SARS-CoV-2Vaccination of the general population was initiated in December 2020 and on January 28, 2021 the first case of deltoid uptake in the 18F-FDG-PET/CT study was reported in relation to the injection site and reactive ipsilateral axillary adenopathies, warning of a potential artifact that massive immunization may have on the interpretation of imaging studies83. Later, an analysis of 728 patients revealed the presence of hypermetabolic adenopathies in 36.4% of the patients who had received a single dose of the Pfizer-BioNTech (BNT162b2) vaccine and in 53.9% of those who had completed the vaccine schedule. In the majority of the cases the lymph nodes were not pathological in size (86%) and were localized at axillary level I ipsilateral to the injection site (99%), although lymph nodes in axillary levels II and II and interpectoral, and less frequently, supraclavicular were also detected. Despite the experience in the reading of PET/CT studies, in up to 14.8% of the cases, the interpretation of these lymph nodes may not be conclusive84.
To date, only one case of systemic inflammatory response has been described after having received the Moderna mRNA-1273 vaccine, visualizing uptake in the deltoid and ipsilateral axillary lymph node in the 18F-FDG-PET/CT in addition to diffuse increase of glucidic metabolism in the spleen85.
A proportional inverse relationship has been found between age and the presence of immunocompromise or hematological disease and the presence of these lymph nodes. Thus, the percentage of lymph node uptake in immunocompromised patients is reduced to 33%86. These factors cannot be acted upon, but an inverse relationship between the intensity of the lymph node uptake and the time since vaccination has been demonstrated. Therefore, to minimize the possibility of artifacts in lymph node interpretation, temporal windows have been proposed for the planning of imaging studies. A 5-day period after the first vaccine dose has been suggested in which the probability of lymph node uptake would be low, and 2–3 weeks after the first or second dose, being preferable at 4–6 weeks84,87. Nevertheless, persistence of lymph node uptake has been described in up to 29% of cases at 7–10 weeks post-vaccination88. In addition to the time factor, in oncological patients with tumors showing laterality (breast cancer, melanoma, sarcoma, head and neck tumors and lung, among others), it would obviously be beneficial to vaccinate in the arm contralateral to the theoretical territory of tumor drainage. And, of course, access to the information of the date and site of vaccination contributes to better interpretation of the findings. In cases in which uncertainty remains, an axillary ultrasound performed 4 weeks after the PET/CT study could contribute to lymph node characterization89. Lastly, not only PET/CT with 18F-FDG shows hypermetabolic axillary lymph nodes. Up to 50% of studies with 68Ga-DOTATATE are positive at the axillary level, with a much lower frequency with 11C-choline, 18F or 68Ga-PSMA and 18F-DOPA86,90 (Fig. 5).
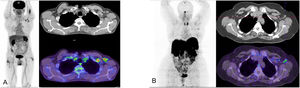
(A) 18F-FDG PET/CT study in a patient who had received the first dose of Vaxzevria 5 days previously. The MIP image shows focal uptake in the area of the injection site in the left arm and ipsilateral axillary adenopathies localized at levels I and II in the axial CT and PET/CT slices.
(B) 68Ga-edotreotide (Somakit-TOC) PET/CT study in a patient who had received the second dose of Vaxzevria 15 days previously. The MIP image shows uptake in the left axilla corresponding to adenopathies at the left axillary level I, visible in the axialCT and PET/CT slices.
Infection by SARS-COV-2 is a systemic process which goes beyond involvement of the respiratory tract. The complications and sequelae of COVID-19 are very varied and increasingly more numerous, and patients require a multidisciplinary approach not only in the acute phase of the disease but also post-COVID.
The role of 18F-FDG-PET/CT in the acute phase is limited by the risk of exposure to the virus during prolonged studies in patients with viral load. Nonetheless, this phase may provide fundamental information for the study of associated complications such as infectious endocarditis.
In the post-COVID state it is too early to draw conclusions, but studies with 18F-FDG-PET/CT can be a useful tool to detect or rule out severe concomitant processes. Alterations of brain metabolism shown with this technique may be a marker of the systemic process.
Lastly, during the next months we are going to live with local immunological response provoked by the vaccine, and therefore it will be fundamental to know the history of each patient with respect to the date and site of injection to adequately program and interpret 18F-FDG-PET-CT studies.
Please cite this article as: Rodríguez-Alfonso B, Ruiz Solís S, Silva-Hernández L, Pintos Pascual I, Aguado Ibáñez S, Salas Antón C. 18F-FDG-PET/TC ante la infección por SARS-CoV-2 y sus secuelas. Rev Esp Med Nucl Imagen Mol. 2021;40:299–309.