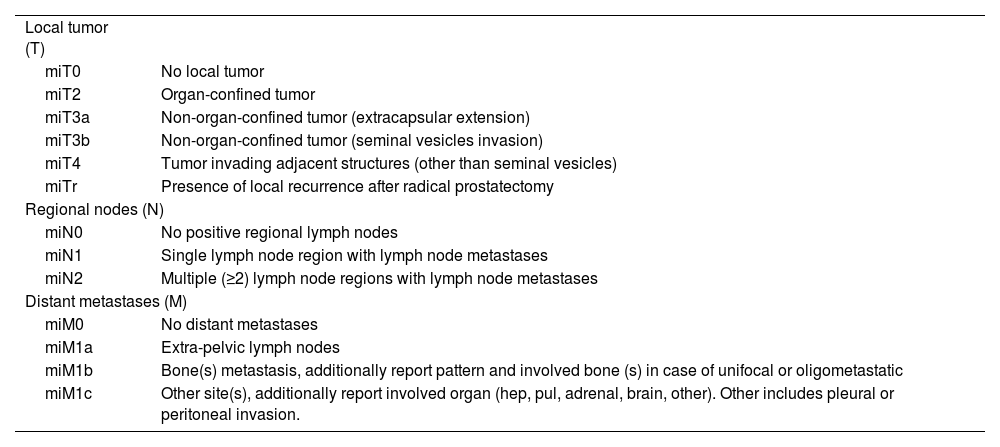
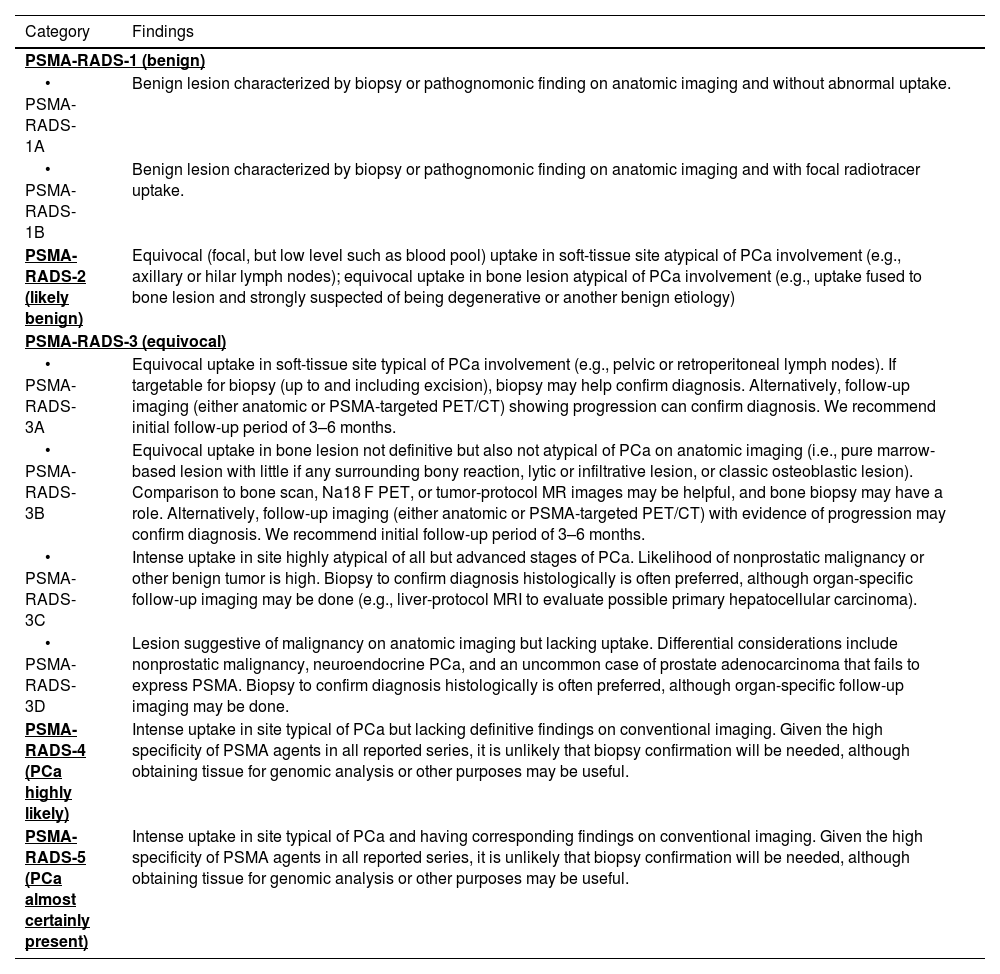
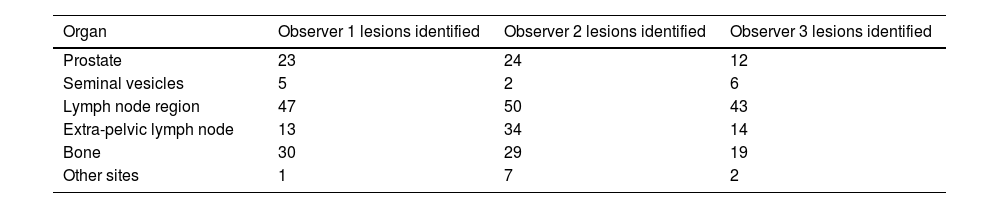
The aim of this study was to determine the agreement between three observers with different levels of experience using the PSMA-RADS 2.0 criteria and the miTNM system for the interpretation of PET-PSMA with [18F]DCFPyL in males with prostate cancer.
Materials and MethodsPET-PSMA images from 114 prostate cancer patients were blindly reported twice by three different observers at intervals of 8 weeks. The evaluations were performed according to the molecular imaging TNM (miTNM) and PSMA-RADS 2.0 criteria. We used Fleiss’ Kappa to analyse inter and intraobserver agreements.
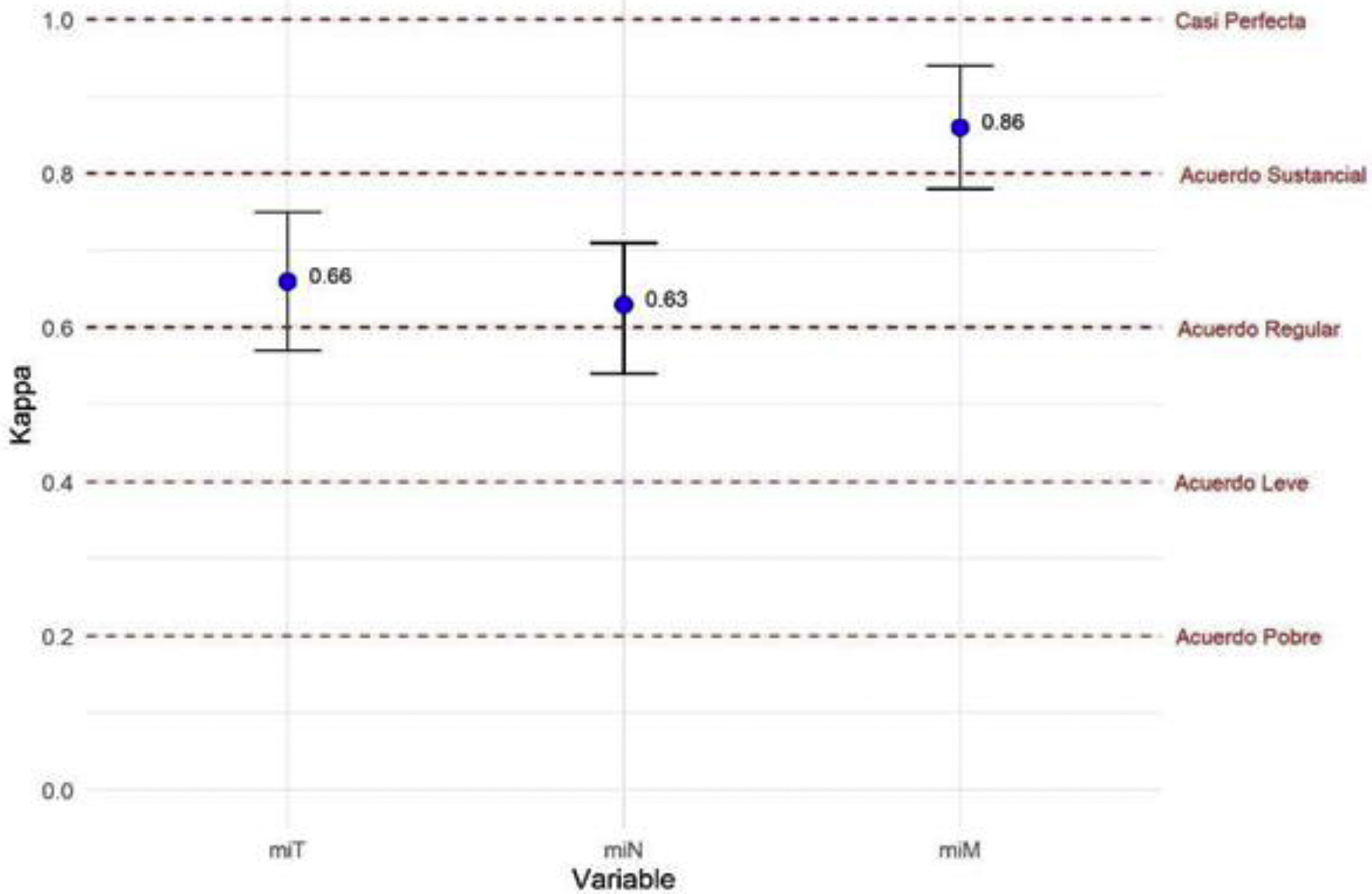
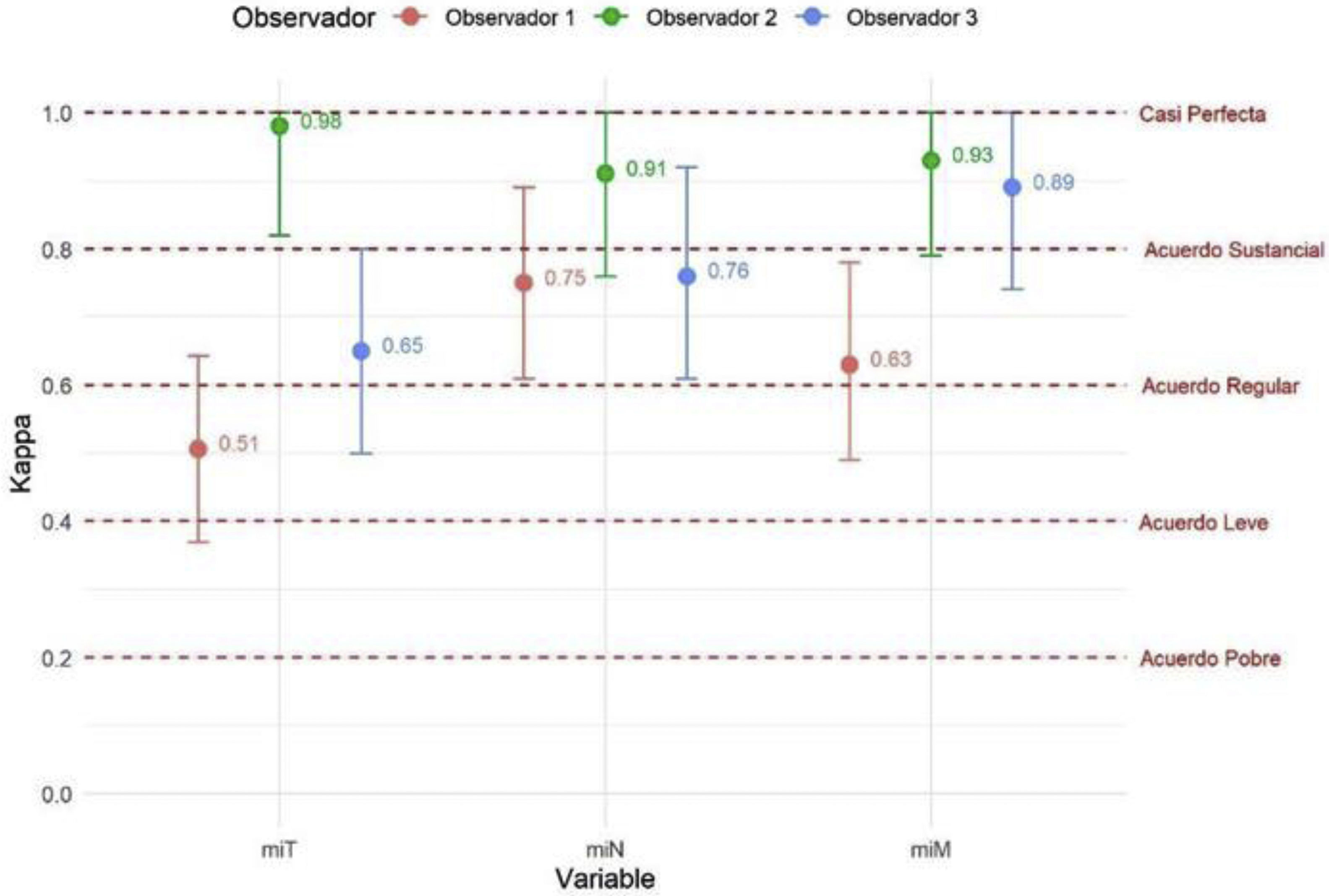
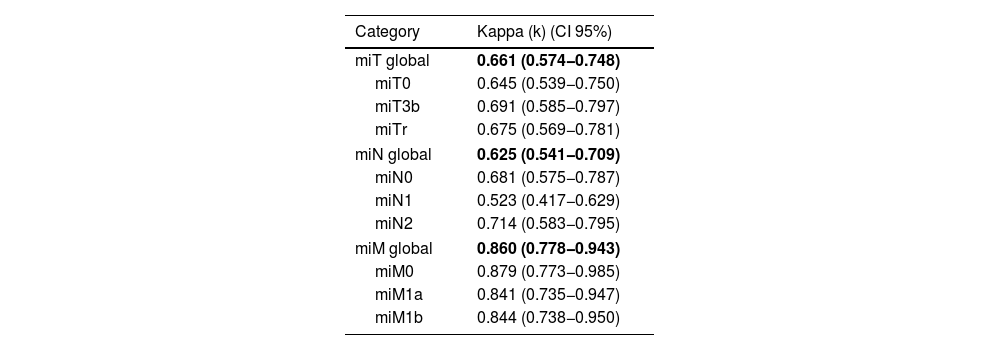
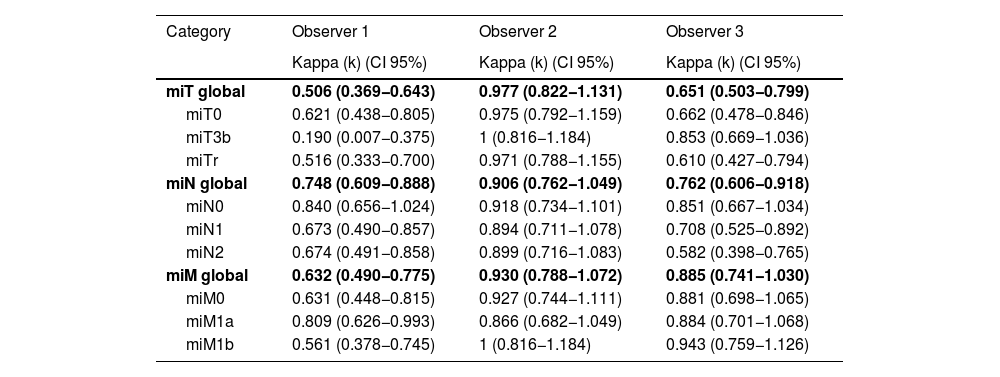
ResultsModerate overall agreement was obtained in the assessment of the PET-PSMA results (Fleiss'k = 0.53; 95% CI 0.45−0.62; p < 0.001), with significant agreement in the miT, miN and miM reports. There was a substantial level of agreement in the reporting of prostatic disease and lymphatic involvement (Fleiss'k = 0.66 and 0.65), being lower than that observed in the reporting of metastatic disease (Fleiss'k = 0.86), especially in the M0 group (Fleiss'k = 0.99). Upon re-evaluation of the images, observer 1 had moderate overall agreement for miT (Fleiss'k = 0.51) and substantial agreement for miN and miM (Fleiss'k 0.75 and 0.63, respectively).
ConclusionsThe use of a structured scoring system such as PSMA-RADS 2.0, as well as the miTNM classification system in the interpretation of PET-PSMA images in prostate cancer patients, provides a highly reproducible report format. High levels of interobserver and intraobserver agreement are found, especially when ruling out disease, which supports its use in routine clinical practice.
El [18F]DCFPyL es un radiofármaco dirigido al antígeno prostático específico de membrana (PSMA) cada vez más extendido en la práctica clínica. El objetivo fue evaluar la concordancia entre tres médicos nucleares con distintos niveles de experiencia en estudios PET-PSMA, según los criterios actualizados PSMA-RADS 2.0 y el TNM molecular.
Material y métodosSe analizaron 114 hombres con CaP a los que se les realizó un PET-PSMA por sospecha de recidiva o para estadificación inicial, con niveles de PSA entre 0.2 y 2 ng/mL. Las imágenes fueron evaluadas por los tres profesionales, cegados a los resultados y con al menos 8 semanas de separación. Se calculó el índice de Kappa para la concordancia inter e intraobservador.
ResultadosSe obtuvo una concordancia global moderada (Kappa = 0.53; p < 0.001), con un acuerdo significativo en la valoración de miTNM tanto intra como interobservador. Hubo un acuerdo sustancial en la evaluación de la afectación prostática y linfática (Kappa = 0.66 y 0.65), y un acuerdo casi perfecto en la evaluación de la afectación metastásica (Kappa = 0.86) especialmente en aquellos pacientes sin enfermedad (Kappa = 0.99 cuando M0). En la reevaluación de las imágenes, se encontró una reproducibilidad de moderada a muy buena en los 3 lectores, sin evidencia de peores resultados de concordancia en el profesional menos experimentado.
ConclusiónEl uso de sistemas estructurados como los criterios PSMA-RADS 2.0 y miTNM para la interpretación de imágenes PET-PSMA presenta una alta reproducibilidad, incluso en médicos nucleares menos experimentados. Se han encontrado valores de concordancia especialmente buenos a la hora de descartar enfermedad.
Article
Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)