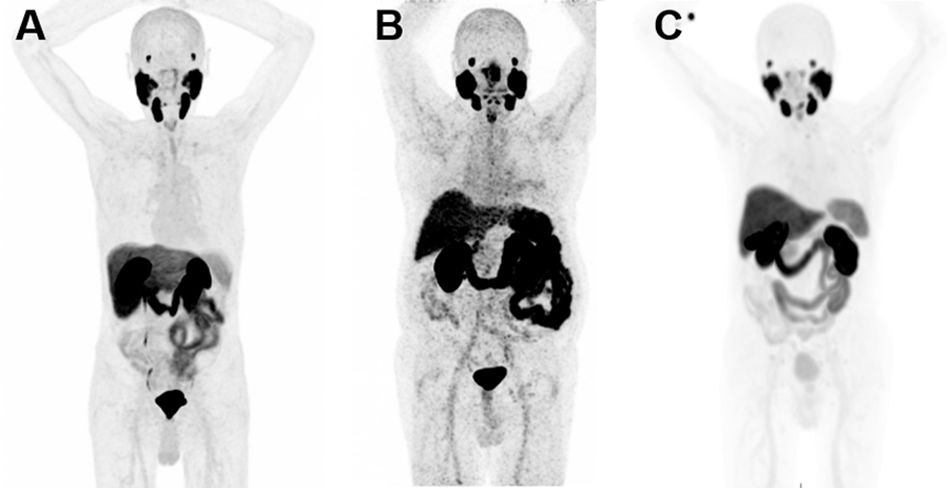
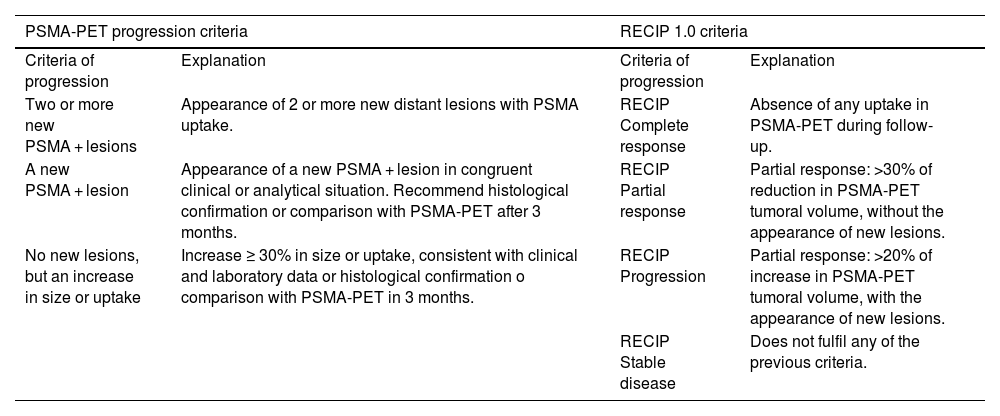
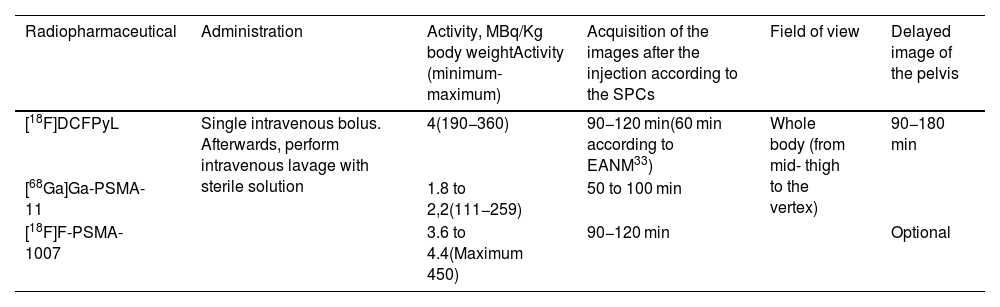
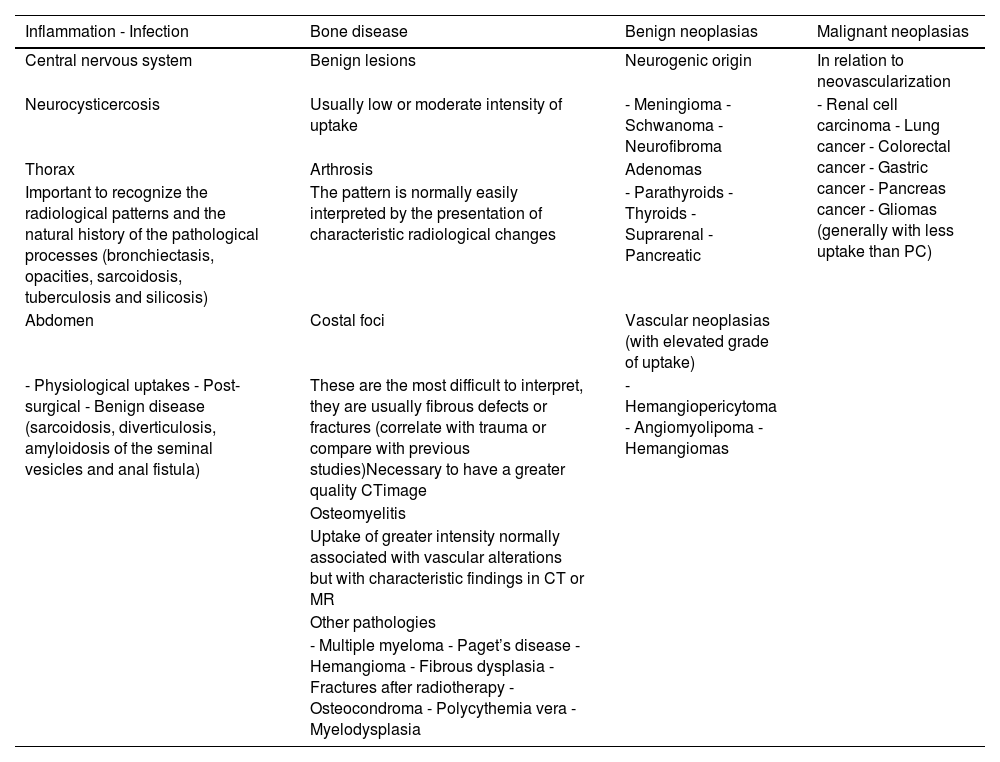
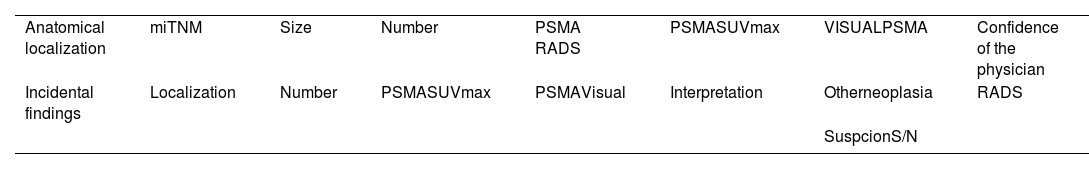
The application of PET/CT with radiopharmaceuticals targeting PSMA is significantly transforming the diagnostic and therapeutic strategies of patients with prostate cancer. In Spain, the availability and access to positron-emitting radiopharmaceuticals targeting Prostate-Specific Membrane Antigen (PSMA) have significantly changed in recent months. These changes are affecting their use in diagnostic procedures. As a result, its use within diagnostic protocols for patients with prostate cancer is undergoing significant modifications. In this collective and cooperative document, the authors have selected the most robust evidence accumulated to date to generate a clinical guide to achieve appropriate use of this technology. A format that presents the most frequent clinical situations and the patient profiles in which PSMA PET/CT plays a significant role or will do so in the immediate future has been chosen. It should be taken into account that regulatory restrictions mediate the current indications for its use in Spain, as well as its current cost and the production capacity of radiopharmaceuticals. The guideline presents a review of the established methodology for optimized imaging with each of the radiopharmaceutical variants targeting PSMA and recommendations for structured and accurate reporting of metabolic findings in combination with CT.
La aplicación de la PET/TC con radiofármacos dirigidos al PSMA está transformando notablemente las estrategias diagnósticas y terapéuticas de los pacientes con cáncer de próstata. En España, la disponibilidad y el acceso a los radiofármacos emisores de positrones dirigidos al antígeno de membrana específico de la próstata (PSMA) han experimentado cambios significativos en los últimos meses. Como resultado, su uso dentro de los protocolos diagnósticos para pacientes con cáncer de próstata está sufriendo modificaciones significativas. En este documento colectivo y cooperativo, los autores han seleccionado la evidencia más robusta acumulada hasta el momento para generar una guía clínica orientada a alcanzar un uso apropiado de esta tecnología. Se ha escogido un formato que presenta las situaciones clínicas más frecuentes y los perfiles de pacientes en los que la PET-PSMA juega un papel trascendente, o lo hará en un futuro próximo. Debe tenerse en cuenta que las indicaciones actuales de su uso en España están mediatizadas por restricciones regulatorias, por su coste actual y por la capacidad de producción de los radiofármacos. La guía presenta una revisión de la metodología consolidada para la obtención optimizada de las imágenes con cada una de las variantes radiofarmacéuticas dirigidas al PSMA y recomendaciones para un informe estructurado y preciso de los hallazgos metabólicos en combinación con la TC.
Article
Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)