Exophiala dermatitidis is a dematiaceous fungus known to cause superficial, subcutaneous, cutaneous and deep seated infections, and rarely central line associated bloodstream infection (CLABSI). A case of CLABSI due to E. dermatitidis in an infant is described.
Case reportClinical and laboratory data were extracted from patient's chart and laboratory records. The isolate was identified as E. dermatitidis by phenotypic characterization and sequencing of the ITS and LSU regions of the ribosomal DNA. Medline search was done to review all cases of CLABSI due to E. dermatitidis. Among the azoles tested, posaconazole (0.06mg/l), voriconazole (0.03mg/l) and itraconazole (0.03mg/l) showed very low MICs when compared to fluconazole (4mg/l)
ConclusionsAs we did not found in the literature any case of CLABSI due to E. dermatitidis in an infant, we report the first one. Sequencing is a mandatory method for accurately identifying this species. Prompt removal of the central line, followed by a treatment with amphotericin B or an azole, seems to be the most effective treatment.
Exophiala dermatitidis es un hongo dematiáceo conocido por causar infecciones superficiales, subcutáneas, cutáneas y profundas, y rara vez infección del torrente sanguíneo asociada a catéter central (central line associated bloodstream infection [CLABSI]). Se describe un caso de CLABSI debido a E. dermatitidis en un bebé.
Caso clínicoLos datos del paciente se extrajeron de la historia clínica y de los registros de laboratorio. El aislamiento se identificó como E. dermatitidis mediante caracterización fenotípica y la secuenciación de las regiones ITS y LSU del ADN ribosómico. Se realizó una búsqueda en Medline para revisar todos los casos de CLABSI debidos a E. dermatitidis. Entre los azoles evaluados, el posaconazol (0,06mg/l), el voriconazol (0,03mg/l) y el itraconazol (0,03mg/l) mostraron valores de MIC muy bajos en comparación con el fluconazol (4mg/l).
ConclusionesTras la revisión de todo lo publicado en la literatura, presentamos el primer caso de CLABSI debido a E. dermatitidis en un lactante. La secuenciación es necesaria para identificar con precisión esta especie. La retirada inmediata del catéter venoso central seguida de un tratamiento con anfotericina B o un azol es el tratamiento más efectivo.
Exophiala dermatitidis is a dark-pigmented yeast-like organism with a worldwide distribution, and has been found in the environment and wild animals.22 It is a well-known cause of local and disseminated pheohyphomycosis in immunocompromised patients but is an uncommon cause of fungemia.17E. dermatitidis is also known to colonize the airways of patients with cystic fibrosis.10 In this report we describe a case of central line associated bloodstream infection (CLABSI) due to E. dermatitidis in a two-month-old female child with suspected mitochondriopathy and an inborn error of metabolism.
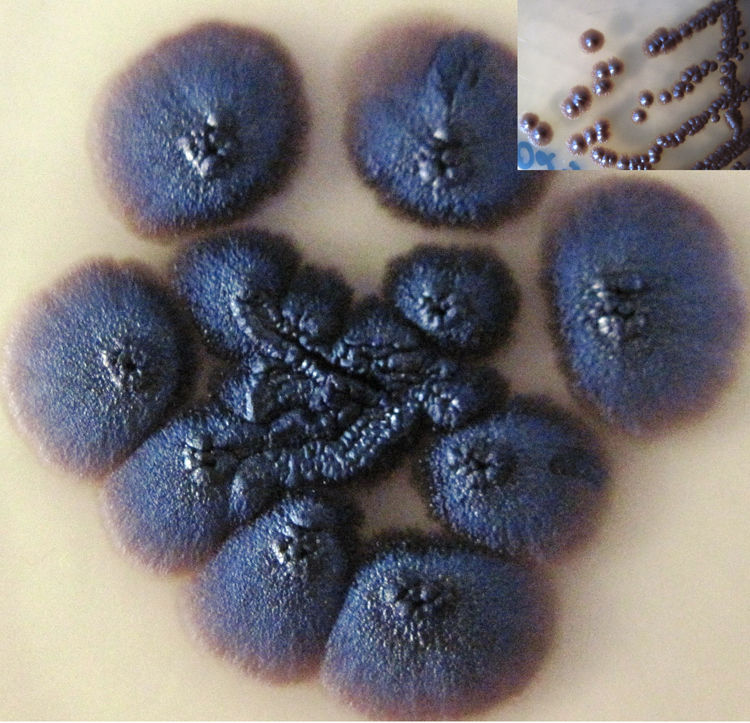
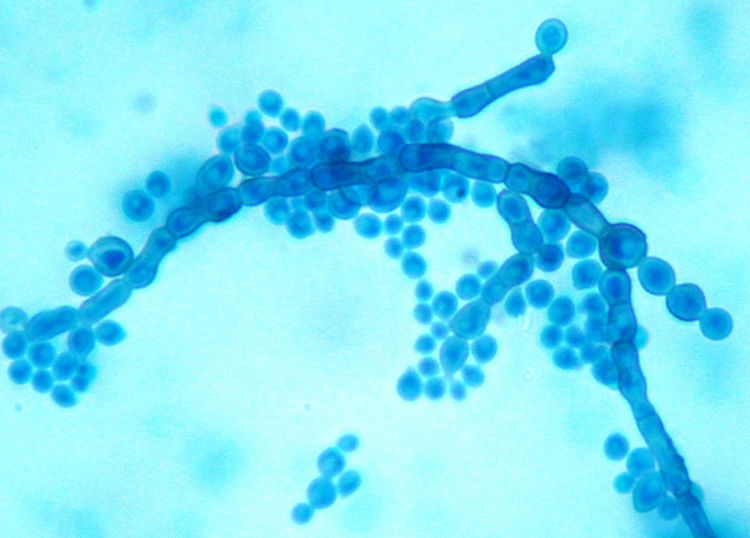
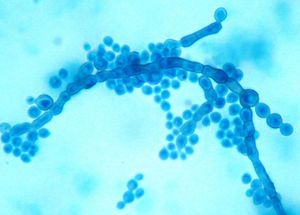
Case reportA two-month-old female infant, born near term, small for gestational age with failure-to-thrive and developmental delay, was admitted with bronchopneumonia, septicaemia and multi-organ dysfunction. Blood investigations showed increased concentrations of lactic acid, pyruvate, succinic acid, fumaric acid and ethylmalonic acid, suggestive of a mitochondriopathy and an inborn error of metabolism. Fluorescent in situ hybridization for DiGeorge syndrome was negative. The patient was treated with broad spectrum antibiotics. Paired blood specimens from the central line and peripheral vein were inoculated into BacT/Alert PF aerobic bottles and incubated in a BacT/ALERT 3D (bioMérieux, Marcy l’Etoile, France) system. Both bottles flagged as positive for budding yeast cells, and grew as light gray, yeast-like colonies at 48h, which on further incubation turned black (Fig. 1). Slide cultures prepared on potato dextrose agar at 37°C revealed long, slender conidiophores with clusters of oval conidia at the tips of the phialides, and aggregates of conidia along the sides of the conidiophores (Fig. 2). The isolate grew at 40°C but failed to grow at 42°C. Based on the macroscopic morphology, microscopic appearance and physiological features, the isolate was identified as Exophiala spp. Since the patient had CLABSI, the catheter was removed and fluconazole therapy was started. Clinical, radiological and ophthalmologic evaluation did not reveal any evidence of disseminated fungal infection. Subsequent blood cultures were negative. The patient was discharged from the hospital after 21 days of fluconazole therapy.
The in vitro antifungal susceptibility of the isolate was determined by a broth microdilution test performed according to the M27-A3 CLSI document.9Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control strains. The minimum inhibitory concentration (MIC) of amphotericin B, fluconazole, voriconazole, itraconazole, posaconazole, flucytosine, caspofungin, micafungin and anidulafungin were 1, 4, ≤0.03, ≤0.03, 0.06, 4, 2, 2 and 4μg/ml respectively.
To confirm the identification, one representative isolate from blood was subjected to sequencing of the internal transcribed spacer (ITS) and the large ribosomal subunit (LSU) D1/D2 regions.27 Both strands of the amplified DNA were sequenced on an ABI 3130XL Genetic analyzer using the BigDye Terminator Kit. Sequences were aligned using the Sequencing Analysis 5.3.1. Searches with GenBank basic local alignment search tool (BLAST) were done for species identification. ITS and LSU sequences of our strain revealed 100% identity with the standard strain of E. dermatitidis (CBS 748.88; accession AF050270.1 and CBS 129657; accession MH 876940.1). The nucleotide sequences of ITS and LSU regions were deposited in GenBank with the accession numbers KF996500 and KF996499, respectively.
DiscussionE. dermatitidis is a melanized yeast-like fungus that produces phialides without collarettes and belongs to the ascomycete order Chaetothyriales.6 This species is distributed worldwide and has been isolated from several environmental sources like sauna facilities, humidifiers, sludge of bathroom pipes and bathwater.1 Colonies are darkly pigmented because of the presence of dihydroxynaphthalene melanin.1 Microscopically E. dermatitidis shows budding yeast cells at first, but pseudohyphae, moniliform hyphae, true hyphae, and sclerotic forms can also be observed. Smears from the same colony show a mix of yeasts and hyphal forms.7,23 The switch between morphotypes can be activated by several environmental factors.7E. dermatitidis is known to cause localized and disseminated phaeohyphomycosis; reports of fungaemia are uncommon.25 Systemic phaeohyphomycosis caused by E. dermatitidis includes respiratory, intestinal, cardiac, and cerebral diseases.16,22 This species has often been reported as a colonizer of the respiratory tract in patients with cystic fibrosis and is occasionally involved in fungal pneumonia and pulmonary phaeohyphomycosis.10 Asymptomatic carriage in the gastrointestinal tract has also been documented.8 Localized infections are due to traumatic implantation into the skin without dissemination into deep organs.8,22 Alternatively, acquisition can be due to inhalation and rarely due to hematogenous seeding.8,18 High mortality rates (80%) due to a disseminated infection by E.dermatitidis have been reported; the spreading may be attributed to its neurotropic behavior and the high MICs to conventional antifungal agents like fluconazole.8,16,18
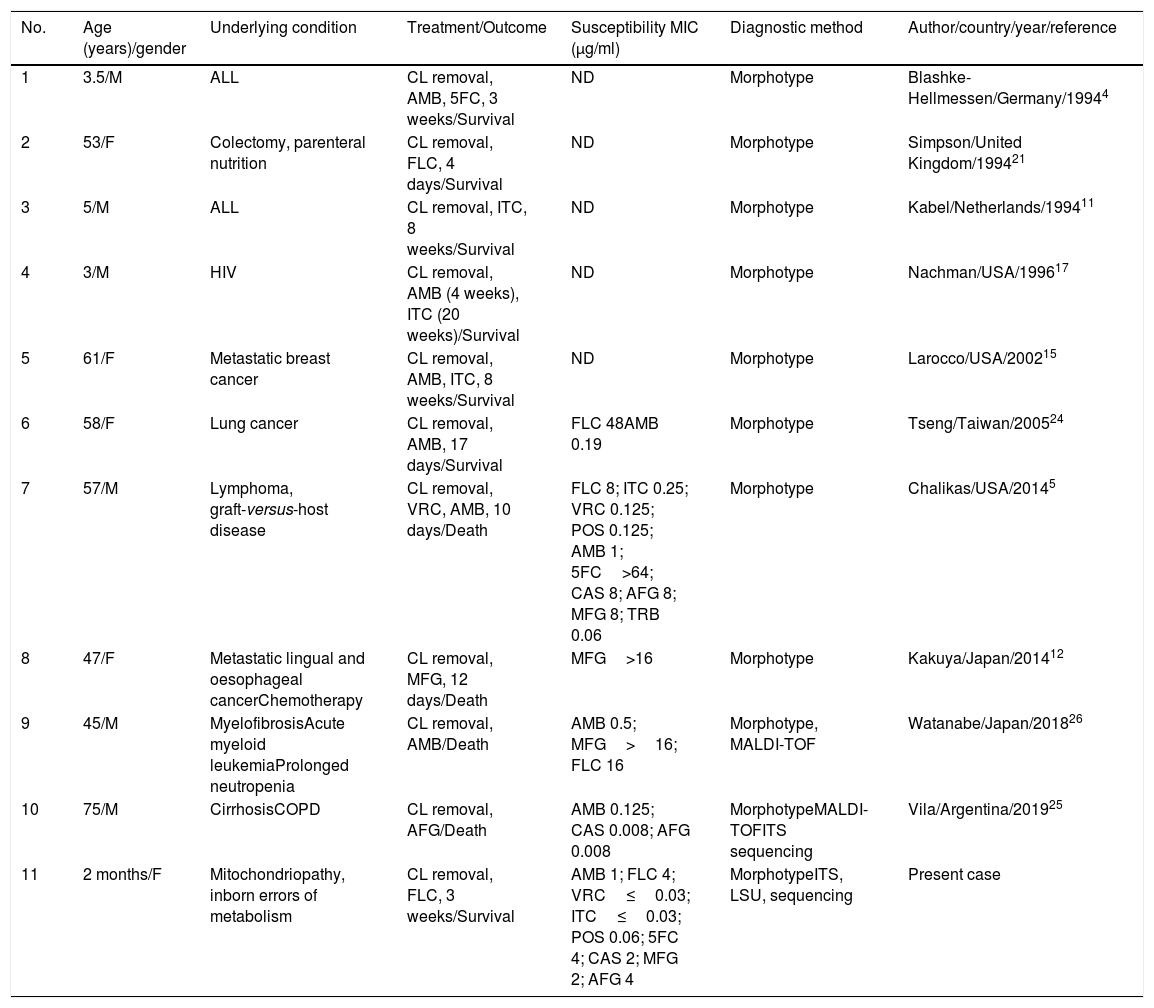
Searching in the Medline database for published articles using the keywords ‘Exophialadermatitidis’, ‘E. dermatitidis and infection’, ‘Wangielladermatitidis’, ‘W. dermatitidis and infection’, ‘CLABSI’, ‘central venous catheter’, ‘catheter-associated’ and ‘nosocomial’, was done. A total of ten cases, whose details are summarized in Table 1, were found. Adding up our case, four patients out of eleven (36.36%) were pediatric. CLABSI due to E.dermatitidis in an infant is being reported for the first time. A solitary case of CLABSI due to Exophialaoligosperma in a three year old leukemic child was reported from Kuwait.2 All the patients had underlying risk factors, the central line was removed, and were treated with systemic antifungals. Most of the patients received amphotericin B with or without an azole with 66.6% (4/6) survival. Overall mortality in patients with CLABSI due to E. dermatitidis was 40% (4/11) and was seen exclusively in adult patients. Though E. dermatitidis is believed to have higher MICs to fluconazole, two cases (including the present one) responded well to monotherapy with fluconazole. Notably, all patients on echinocandins died. Withdrawal of central line and the administration of antifungals are the mainstay of the treatment in cases of central line associated fungemia.25 The increase in the incidence of CLABSI due to E. dermatitidis can be attributed to its propensity to form biofilms.14 A review of 40 cases from 1990 to 2011 by Patel et al. found that 70% of the cases had an identifiable underlying condition.18 Steroid therapy, cystic fibrosis, cancer, transplantation, intravenous drug abuse and the use of long-term catheter were risk factors for the disseminated disease.18 They also reported that cerebral infection proved fatal in 90% of the cases and had the highest mortality.18 The source of the infection in our case could not be identified. Since there were no other cases during this period an outbreak was ruled out.
Summary of the published cases of E. dermatitidis CLABSI excluding outbreaks.
| No. | Age (years)/gender | Underlying condition | Treatment/Outcome | Susceptibility MIC (μg/ml) | Diagnostic method | Author/country/year/reference |
|---|---|---|---|---|---|---|
| 1 | 3.5/M | ALL | CL removal, AMB, 5FC, 3 weeks/Survival | ND | Morphotype | Blashke-Hellmessen/Germany/19944 |
| 2 | 53/F | Colectomy, parenteral nutrition | CL removal, FLC, 4 days/Survival | ND | Morphotype | Simpson/United Kingdom/199421 |
| 3 | 5/M | ALL | CL removal, ITC, 8 weeks/Survival | ND | Morphotype | Kabel/Netherlands/199411 |
| 4 | 3/M | HIV | CL removal, AMB (4 weeks), ITC (20 weeks)/Survival | ND | Morphotype | Nachman/USA/199617 |
| 5 | 61/F | Metastatic breast cancer | CL removal, AMB, ITC, 8 weeks/Survival | ND | Morphotype | Larocco/USA/200215 |
| 6 | 58/F | Lung cancer | CL removal, AMB, 17 days/Survival | FLC 48AMB 0.19 | Morphotype | Tseng/Taiwan/200524 |
| 7 | 57/M | Lymphoma, graft-versus-host disease | CL removal, VRC, AMB, 10 days/Death | FLC 8; ITC 0.25; VRC 0.125; POS 0.125; AMB 1; 5FC>64; CAS 8; AFG 8; MFG 8; TRB 0.06 | Morphotype | Chalikas/USA/20145 |
| 8 | 47/F | Metastatic lingual and oesophageal cancerChemotherapy | CL removal, MFG, 12 days/Death | MFG>16 | Morphotype | Kakuya/Japan/201412 |
| 9 | 45/M | MyelofibrosisAcute myeloid leukemiaProlonged neutropenia | CL removal, AMB/Death | AMB 0.5; MFG>16; FLC 16 | Morphotype, MALDI-TOF | Watanabe/Japan/201826 |
| 10 | 75/M | CirrhosisCOPD | CL removal, AFG/Death | AMB 0.125; CAS 0.008; AFG 0.008 | MorphotypeMALDI-TOFITS sequencing | Vila/Argentina/201925 |
| 11 | 2 months/F | Mitochondriopathy, inborn errors of metabolism | CL removal, FLC, 3 weeks/Survival | AMB 1; FLC 4; VRC≤0.03; ITC≤0.03; POS 0.06; 5FC 4; CAS 2; MFG 2; AFG 4 | MorphotypeITS, LSU, sequencing | Present case |
ND: not done; CL: central line; HIV: human immunodeficiency virus; ALL: acute lymphocytic leukemia; COPD: chronic obstructive pulmonary disease; AMB: amphotericin B; ITC: itraconazole; FLC: fluconazole; VRC: voriconazole; 5FC: flucytosine; MFG: micafungin; POS: posaconazole; CAS: caspofungin; AFG: anidulafungin; TRB: terbinafine; MALDI-TOF: matrix-assisted laser desorption/ionization-time of flight; ITS: internal transcribed spacer; LSU: large ribosomal subunit.
The phenotypic characteristics that differentiate E. dermatitidis from other species of the genus are its ability to grow at 42°C, inability to utilize nitrite or nitrate and the absence of annelides on microscopy.18 The monoclonal antibody used in Pastorex Aspergillus antigen test for the detection of Aspergillus galactomannan antigen may cross-react with E. dermatitidis.13 The differentiation of E. dermatitidis from other species of Exophiala, like Exophiala phaeomuriformis, solely on the basis of morphological features is difficult. ITS1 sequencing should be used to differentiate closely-related black yeast species.2,14,18,25 Recent studies have found that matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) can also accurately identify E. dermatitidis.3,25
E. dermatitidis has been found to have high MICs to fluconazole and 5-fluorocytosine, whereas amphotericin B, itraconazole, voriconazole and posaconazole seem to be more effective in vitro.19 In the present case, the isolate was susceptible to amphotericin B, voriconazole, itraconazole and posaconazole, and had a higher MIC of fluconazole in comparison with other azoles tested. The isolate was non-susceptible to 5-fluorocytosine and echinocandins. Treatment of localized lesions includes complete surgical excision followed by antifungal therapy with amphotericin B or voriconazole. Monotherapy with echinocandins has been reported to be suboptimal.25,26 However, in case of systemic infection and involvement of the central nervous system, addition of amphotericin B to the treatment regimen is recommended. Posaconazole has the broadest spectrum of any oral agent and can be considered in cases of central nervous system disease.20
We report the first case of CLABSI due to E. dermatitidis in an infant with a review of all cases of CLABSI published in literature. Sequencing was found to be a reliable method to accurately identify this species. Prompt removal of the central line followed by treatment with amphotericin B or an azole seems to be the most effective treatment.
FundingNone declared.
Conflict of interestWe certify that there are no entities with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.