The mucoralean fungi are emerging causative agents of primary cutaneous infections presenting in the form of necrotizing fasciitis.
AimsThe aim of this study was to investigate a series of suspected necrotizing fasciitis cases by Apophysomyces species over one-year period in a northern Indian hospital.
MethodsThe clinical details of those patients suspected to suffer from fungal necrotizing fasciitis were recorded. Skin biopsies from local wounds were microscopically examined and fungal culturing was carried out on standard media. The histopathology was evaluated using conventional methods and special stains. Apophysomyces isolates were identified by their morphology and by molecular sequencing of the internal transcribed spacer (ITS) region of the ribosomal genes. Antifungal susceptibility testing was carried out following EUCAST guidelines and treatment progress was monitored.
ResultsSeven patients were found to be suffering from necrotizing fasciitis caused by Apophysomyces spp. Six isolates were identified as Apophysomyces variabilis and one as Apophysomyces elegans. Five patients had previously received intramuscular injections in the affected area. Three patients recovered, two died and the other two left treatment against medical advice and are presumed to have died due to their terminal illnesses. Posaconazole and terbinafine were found to be the most active compounds against A. variabilis, while the isolate of A. elegans was resistant to all antifungals tested.
ConclusionsApophysomyces is confirmed as an aggressive fungus able to cause fatal infections. All clinicians, microbiologists and pathologists need to be aware of these emerging mycoses as well as of the risks involved in medical practices, which may provoke serious fungal infections such as those produced by Apophysomyces.
Los hongos mucorales son agentes emergentes causantes de infecciones cutáneas primarias presentes en forma de fascitis necrotizante.
ObjetivosLa finalidad de este estudio fue la de investigar una serie de infecciones sugestivas de fascitis necrotizante causadas por alguna de las especies de Apophysomyces a lo largo de un año en un hospital del norte de la India.
MétodosSe obtuvieron los datos de todos los pacientes con sospecha de fascistis necrotizante. Las biopsias de piel de la zona afectada fueron cultivadas en medios de cultivos estándar y se evaluaron histopatológicamente mediante tinciones convencionales y específicas para hongos. Los aislamientos de Apophysomyces fueron identificados morfológicamente y mediante la secuenciación del espaciador intergénico ribosomal (ITS). La sensibilidad antifúngica se determinó mediante el método EUCAST y la evolución de los pacientes fue monitorizada.
ResultadosSe encontraron siete pacientes con fascitis necrotizante causada por especies de Apophysomyces. Seis aislamientos fueron identificados como Apophysomyces variabilis y uno como Apophysomyces elegans. Cinco pacientes habían recibido previamente inyecciones intramusculares en el área afectada. Tres pacientes se recuperaron, dos fallecieron y de los dos restantes no se tiene seguimiento médico, aunque presumiblemente fallecieron debido a que padecían enfermedades terminales. El posaconazol y la terbinafina fueron los compuestos más activos frente a A. variabilis, mientras que el único aislamiento de A. elegans fue resistente a todos los antifúngicos ensayados.
ConclusionesSe confirma que Apophysomyces es un hongo agresivo capaz de causar infecciones con desenlace fatal. Clínicos, microbiólogos y patólogos deben ser conscientes de los riesgos de estas micosis emergentes y de que determinadas prácticas médicas puedan provocar infecciones fúngicas graves como las producidas por Apophysomyces.
Necrotizing fasciitis, commonly known as flesh-eating disease, is one of the severe soft tissue infections of skin caused by bacterial as well as fungal pathogens. Some fungi of the Mucorales order can produce this rapidly progressive infection, which destroys the soft tissues that cover muscles, spreading along the fascial planes and invading deeper and deeper into the tissues, often being fatal even when treated in a timely manner.16 Most commonly, the organism enters the body through a breach in continuity of skin, like a cut, burn, insect bite or puncture wound. The introduction of contaminated soil at the site of trauma may serve as the primary means of inoculation of this organism. The necrotizing infections are found mainly in immunocompetent patients, as well as in those with or without diabetes mellitus. The infection can also be associated to health-care risks such as post-surgery, intramuscular injections, skin tests and contamination of a plaster of Paris cast applied to reduce fractures, etc.2,4,10,11
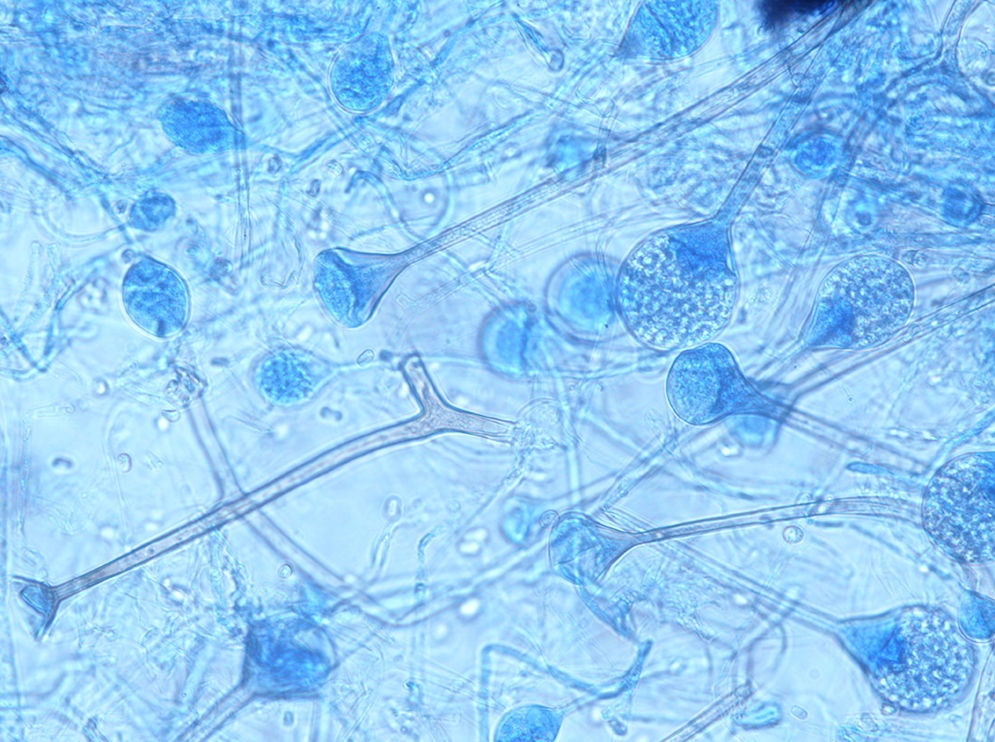
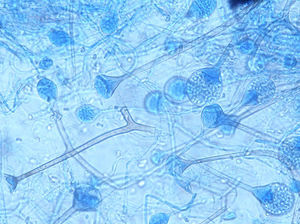
The genus Apophysomyces (family Saksenaeaceae, order Mucorales) is a common cause of such devastating life-threatening infections.3 The species in this genus are widely distributed in soil in tropical and subtropical climates, with clinical cases reported mainly from India, the United States and Australia. The type species of this genus, Apophysomyces elegans, was isolated for the first time in 1979 from two soil samples in a mango orchard in northern India.12 The species of Apophysomyces are thermotolerant and can grow rapidly, even above the body temperature, up to 42°C. The morphology of Apophysomyces in culture is quite similar to that of Lichtheimia species. However, Apophysomyces has dark brown champagne glass-shaped apophyses and a dark, thick-walled region in the sporangiophore just below the apophyses. In addition, Apophysomyces has a foot cell that resembles those produced by Aspergillus species. Recently, based on genetic, physiological and morphological features, three new species have been reported, i.e. Apophysomyces ossiformis, Apophysomyces trapeziformis and Apophysomyces variabilis, the latter being the most common in clinical infections.1,9 The aim of this study was to investigate a series of cases suspected of necrotizing fasciitis caused by Apophysomyces species over one-year period in a northern Indian hospital.
Materials and methodsThis prospective study was conducted over one-year period from September 2010 to August 2011 in the Departments of General Surgery, Microbiology and Pathology at the Government Medical College Hospital, Chandigarh (northern India) in association with the Mycology Unit, Rovira i Virgili University, Reus, and Mycology Department, Spanish National Center for Microbiology, Instituto de Salud Carlos III, Madrid (Spain). Tissue biopsy samples were handled delicately, avoiding fungal cell damage. The presence of mucoralean fungi was investigated, focusing on direct microscopic examination with potassium hydroxide and calcofluor white wet mounts. The histopathology exam was carried out by using hematoxylin and eosin, periodic acid-Schiff and Grocott's methenamine silver stains. Fungal cultures were made on blood agar, Sabouraud's dextrose agar (SDA; HiMedia, Mumbai, India), brain–heart infusion agar (BHIA; HiMedia, Mumbai, India) and oatmeal agar (OMA; HiMedia, Mumbai, India) in duplicate and incubated at 25°C and 37°C. The cultures were examined daily for the presence of Mucorales species, particularly, Apophysomyces spp., characterized by showing rapid growing (2–4 days), white cottony creamy-white colonies becoming pale-yellow on aging, filling almost the entire tube and/or petri dish. The isolates were identified by gross morphology along with microscopic examination on lactophenol-cotton blue (LCB) mounts. All isolates tentatively identified as Apophysomyces species in India were later confirmed phenotypically and molecularly in the reference laboratory at Reus (Spain). These isolates were grown on Czapek-Dox agar (BD Difco, USA) at 37°C for one week, and microscopic features were determined on the sixth day in lactic acid mounts. The capacity to hydrolyse and to assimilate esculin was tested by growing the fungi on bile-esculin agar (BEA; Panreac, Barcelona, Spain) at 37°C for one week.1 The amplification and nucleotide sequencing of the internal transcribed spacer (ITS) region of the ribosomal genes were carried out, and the obtained sequences were compared with those of the type strains of Apophysomyces species.
The antifungal susceptibility testing of the Apophysomyces isolates was carried out using the EUCAST broth microdilution method.15,17 The minimum inhibitory concentration (MIC) was calculated for amphotericin B (AMB), itraconazole (ITC), voriconazole (VRC), ravuconazole (RVC), posaconazole (PSC) and terbinafine (TRB), while minimum effective concentration (MEC) was calculated for caspofungin (CPF) and micafungin (MCF). Growth was examined visually after incubation at 35°C for 24h, and the MICs or MECs were determined. Aspergillus fumigatus ATCC 2004305 and Aspergillus flavus ATCC 2004304 were used as quality control strains.
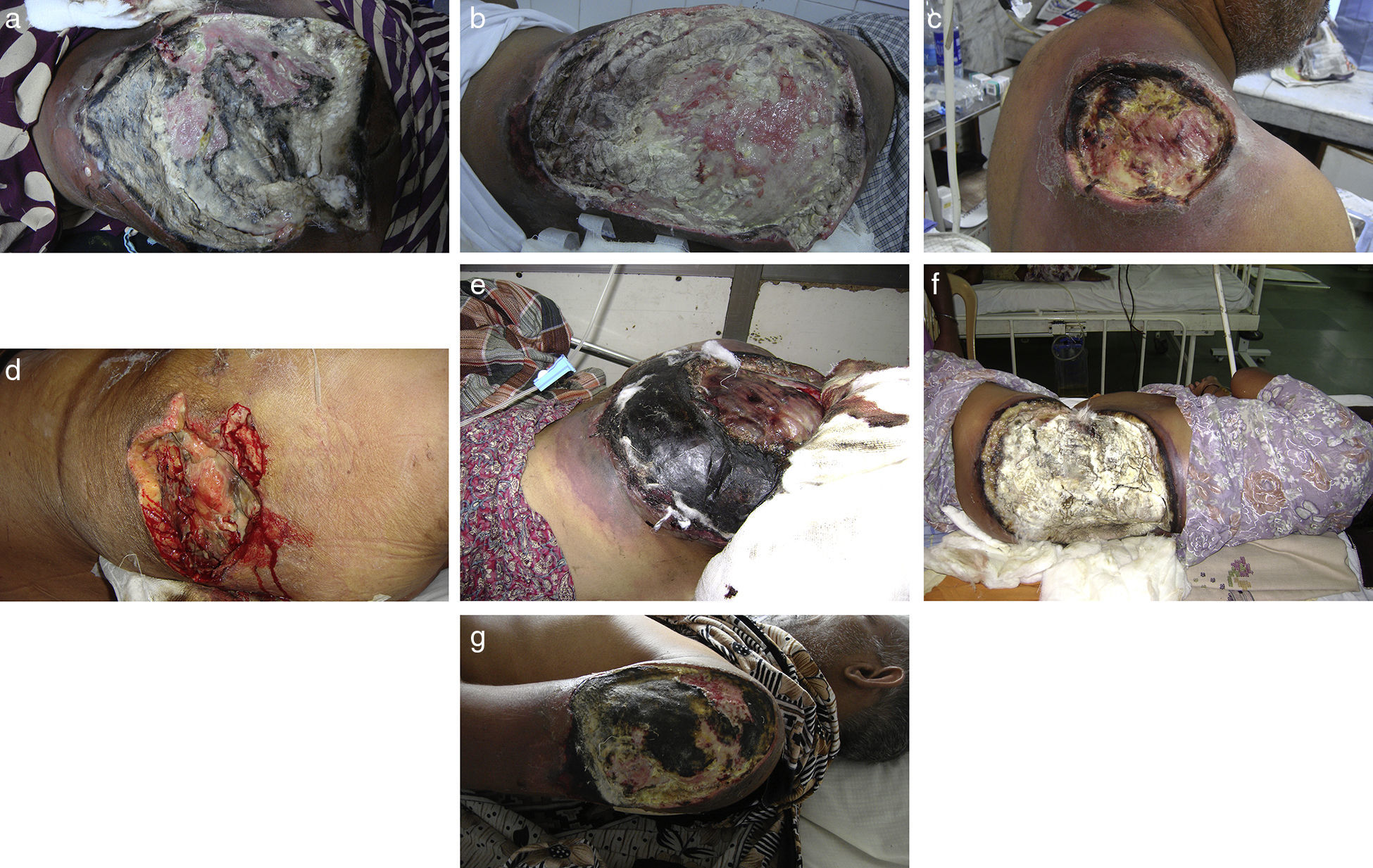
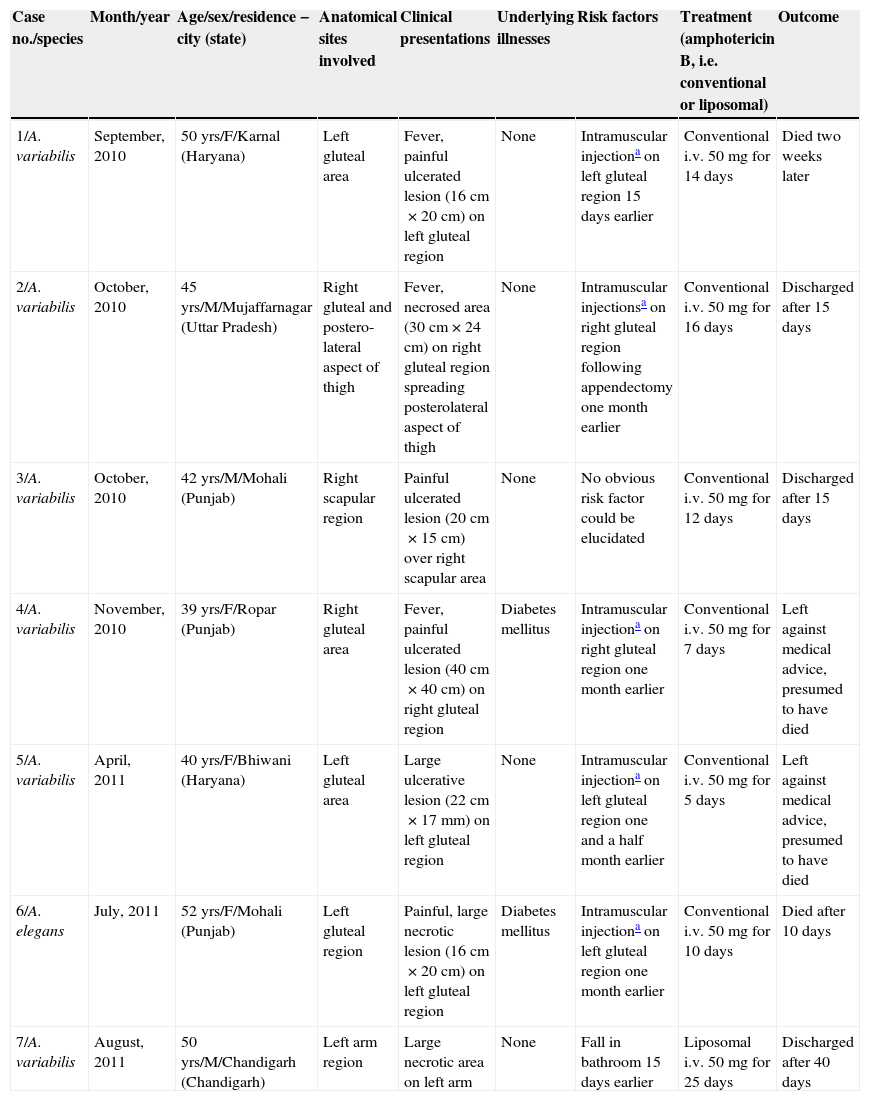
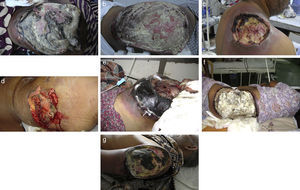
ResultsSeven cases of Apophysomyces infections were detected. The histopathology examination of the biopsy samples was positive in all cases, showing broad, ribbon-like sparsely septate hyphae, consistent with a mucormycosis. The general treatment procedure was identical in all the cases and consisted of local debridement of wounds along with a daily infusion of 50mg of either deoxycholate amphotericin B (six of the seven patients) or liposomal amphotericin B diluted in dextrose 5%. In this study all the isolates of Apophysomyces showed rapid growth on conventional media i.e. SDA, BHIA and OMA, with abundant sporulation even after two days of incubation, thereby not requiring any special technique for their phenotypic identification. Six of the seven isolates were identified phenotypically as A. variabilis and confirmed by ITS sequencing, showing 99–100% of similarity with the type strain of the species (CBS 658.93), and one isolate was identified as A. elegans, showing 100% of similarity with the type strain (CBS 476.78). The GenBank accession number of the nucleotide sequence is HE664070 (749bp long) for A. elegans isolate FMR 12015, and HE664071 (758bp long) for one of the isolates of A. variabilis (FMR 12016). Clinical details and outcome of the patients are shown in Table 1. The lesions of patients and microscopic fungal morphological details are shown in Figs. 1a–g, 2a−c and 3, respectively.
Clinical profile of patients infected by Apophysomyces spp. presented at GMCH, Chandigarh, over one-year period.
| Case no./species | Month/year | Age/sex/residence − city (state) | Anatomical sites involved | Clinical presentations | Underlying illnesses | Risk factors | Treatment (amphotericin B, i.e. conventional or liposomal) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1/A. variabilis | September, 2010 | 50 yrs/F/Karnal (Haryana) | Left gluteal area | Fever, painful ulcerated lesion (16cm×20cm) on left gluteal region | None | Intramuscular injectiona on left gluteal region 15 days earlier | Conventional i.v. 50mg for 14 days | Died two weeks later |
| 2/A. variabilis | October, 2010 | 45 yrs/M/Mujaffarnagar (Uttar Pradesh) | Right gluteal and postero-lateral aspect of thigh | Fever, necrosed area (30cm×24cm) on right gluteal region spreading posterolateral aspect of thigh | None | Intramuscular injectionsa on right gluteal region following appendectomy one month earlier | Conventional i.v. 50mg for 16 days | Discharged after 15 days |
| 3/A. variabilis | October, 2010 | 42 yrs/M/Mohali (Punjab) | Right scapular region | Painful ulcerated lesion (20cm×15cm) over right scapular area | None | No obvious risk factor could be elucidated | Conventional i.v. 50mg for 12 days | Discharged after 15 days |
| 4/A. variabilis | November, 2010 | 39 yrs/F/Ropar (Punjab) | Right gluteal area | Fever, painful ulcerated lesion (40cm×40cm) on right gluteal region | Diabetes mellitus | Intramuscular injectiona on right gluteal region one month earlier | Conventional i.v. 50mg for 7 days | Left against medical advice, presumed to have died |
| 5/A. variabilis | April, 2011 | 40 yrs/F/Bhiwani (Haryana) | Left gluteal area | Large ulcerative lesion (22cm×17mm) on left gluteal region | None | Intramuscular injectiona on left gluteal region one and a half month earlier | Conventional i.v. 50mg for 5 days | Left against medical advice, presumed to have died |
| 6/A. elegans | July, 2011 | 52 yrs/F/Mohali (Punjab) | Left gluteal region | Painful, large necrotic lesion (16cm×20cm) on left gluteal region | Diabetes mellitus | Intramuscular injectiona on left gluteal region one month earlier | Conventional i.v. 50mg for 10 days | Died after 10 days |
| 7/A. variabilis | August, 2011 | 50 yrs/M/Chandigarh (Chandigarh) | Left arm region | Large necrotic area on left arm | None | Fall in bathroom 15 days earlier | Liposomal i.v. 50mg for 25 days | Discharged after 40 days |
(a) Necrotizing fasciitis of left gluteal area (case 1); (b) necrotizing fasciitis of right gluteal area (case 2); (c) necrotizing fasciitis of right scapular region (case 3); (d) necrotizing fasciitis of right gluteal area (case 4); (e) necrotizing fasciitis of left gluteal area (case 5); (f) necrotizing fasciitis of left gluteal area (case 6); and (g) necrotizing fasciitis of left arm region (case 7).
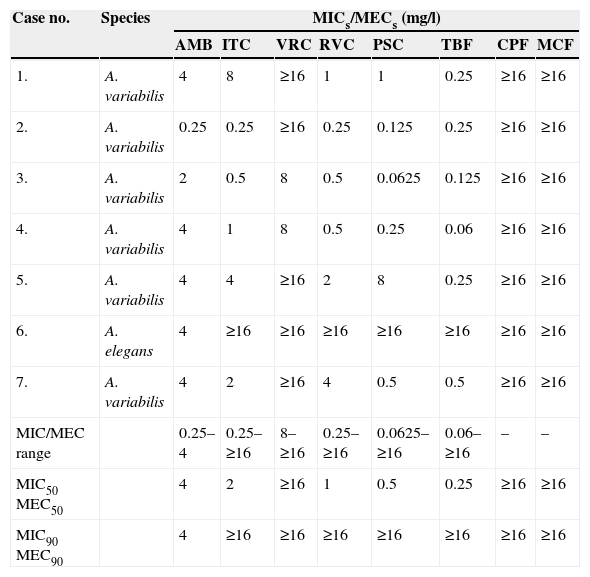
The antifungal susceptibility testing pattern of all seven isolates is shown in Table 2. The MICs of quality control strains used in this study were within the accepted values. PSC and TBF were the most active drugs against the isolates of A. variabilis (MICs, ranging from 0.125 to 0.5mg/l and 0.06 to 1mg/l, respectively) except for one isolate that showed a PSC MIC of 8mg/l. Noteworthy is the isolate of A. elegans, which showed high MICs for all the antifungals tested, with the highest MIC for AMB (4mg/l).
Antifungal susceptibility testing of the Apophysomyces spp. isolates.
| Case no. | Species | MICs/MECs (mg/l) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AMB | ITC | VRC | RVC | PSC | TBF | CPF | MCF | ||
| 1. | A. variabilis | 4 | 8 | ≥16 | 1 | 1 | 0.25 | ≥16 | ≥16 |
| 2. | A. variabilis | 0.25 | 0.25 | ≥16 | 0.25 | 0.125 | 0.25 | ≥16 | ≥16 |
| 3. | A. variabilis | 2 | 0.5 | 8 | 0.5 | 0.0625 | 0.125 | ≥16 | ≥16 |
| 4. | A. variabilis | 4 | 1 | 8 | 0.5 | 0.25 | 0.06 | ≥16 | ≥16 |
| 5. | A. variabilis | 4 | 4 | ≥16 | 2 | 8 | 0.25 | ≥16 | ≥16 |
| 6. | A. elegans | 4 | ≥16 | ≥16 | ≥16 | ≥16 | ≥16 | ≥16 | ≥16 |
| 7. | A. variabilis | 4 | 2 | ≥16 | 4 | 0.5 | 0.5 | ≥16 | ≥16 |
| MIC/MEC range | 0.25–4 | 0.25–≥16 | 8–≥16 | 0.25–≥16 | 0.0625–≥16 | 0.06–≥16 | – | – | |
| MIC50 MEC50 | 4 | 2 | ≥16 | 1 | 0.5 | 0.25 | ≥16 | ≥16 | |
| MIC90 MEC90 | 4 | ≥16 | ≥16 | ≥16 | ≥16 | ≥16 | ≥16 | ≥16 | |
Abbreviations: AMB, amphotericin B; ITC, itraconazole; VRC, voriconazole; RVC, ravuconazole; PSC, posaconazole; TRB, terbinafine; CPF, caspofungin; MCF, micafungin; MIC, minimum inhibitory concentration; MEC, minimum effective concentration; MIC50, MIC causing inhibition of 50% of the isolates; MIC90, MIC causing inhibition of 90% of the isolates.
After publication of 74 cases caused by Apophysomyces species,8 newer additional cases from different parts of the world have been reported, including our seven cases, increasing the total list up to nearly a hundred. A case of A. variabilis invasive wound infection after burn injury has been reported recently in Japan.5 Important attention is drawn to the 13 cases of necrotizing soft tissue infections caused by A. trapeziformis following a F5 tornado that swept through Joplin (Missouri, USA) in May 2011,7,13,18 and an additional case reported 10 months after the tornado.6 This seems to demonstrate the hypothesis of Alvarez et al. that diverse clades of Apophysomyces are prevalent in different parts of the world, i.e. Indian isolates mainly belonged to A. variabilis and American ones to A. trapeziformis.1 We had earlier reported four cases of cutaneous infections by Apophysomyces over a six-year period in our institute (GMCH).4 It is also worth mentioning that no other genera of mucoralean fungi were found to be involved in a set of cases of fungal necrotizing fasciitis over this one-year period.
Although the source of the infection among these patients could not be definitively ascertained, in five patients it appeared to be the result of intramuscular injections by soil-contaminated needles and, in the other patients, traumatic wounds with a secondary infection by the infecting agent. It would be interesting to explore the presence of these fungi in different soils of the regions where the infections appeared.
The possibility of an Apophysomyces infection must be considered in the differential diagnosis in cases of rapidly progressive necrosis of a wound, especially when there is a lack of response to antibacterial chemotherapy in an otherwise healthy patient. The blackening of the wound due to local necrosis, and blood clot deposition due to angioinvasive ability of the fungus is characteristic. Successful treatment requires an early diagnosis and debridement of infected tissues in conjunction with the administration of amphotericin B. A recent murine experimental study has demonstrated the efficacy of PSC in the treatment of disseminated infections by A. variabilis15; however, there is no clinical experience on the use of this drug and its pharmacodynamic and pharmacokinetic properties limit its use in severely ill patients. In two of the three cases where the infection was solved, the isolates showed AMB MICs of 0.25 and 2mg/l. The isolates from the other cases showed AMB MICs of 4mg/l. Although the number of isolates tested is still very low and further studies with more isolates are needed, this seems to indicate that infections by isolates against which AMB shows MICs >2mg/ml would be more difficult to treat. The doses of AMB were lower than the current recommendations that protect patients’ kidney function. Among the three survivors, treatment duration varied from two to four weeks. The use of echinocandins can be considered due to their greater safety and ease of administration6; however, the high MICs showed here by these drugs question this possibility.
Considering that severe infections by Apophysomyces are now being increasingly detected, a high level of clinical suspicion, along with active co-ordination of clinicians, microbiologists and pathologists, is a must for early and correct diagnosis as well as proper management of the infection. Prompt diagnosis is critical, and if valuable time is lost during the initial stage of the disease the patients’ chances of survival become very bleak. The rapid and luxurious mycelial colony on conventional fungal culture media is important as it suggests that at least a mucoralean fungus is growing. It has been reported that Apophysomyces species require special conditions to sporulate, such as growth on Czapek-Dox agar or the use of a water flotation technique14; however, in this study all the strains of Apophysomyces sporulated very well on conventional media. In our institute (GMCH), we are regularly creating awareness of such devastating infections and the importance of timely sampling for fungal culture and histopathology examination. As soon as a patient suffering a necrotizing fasciitis reaches hospital, a KOH test to check the presence of any fungi in the wounds is made. Aggressive debridement is immediately carried out and repeated shortly afterwards, in view of the re-growth of fungi at the borders, until the entire wound is clear and granulation tissue starts appearing. Furthermore, the iatrogenic risk factors are controlled by using proper aseptic techniques.
This study has demonstrated for the first time the involvement of A. elegans, considered for a long time to be the only species of the genus, in a case of human infection. Together with its poor in vitro response to antifungals this species is potentially a serious pathogen. On this occasion it caused the death of the patient.
Conflict of interestThis is to certify that none of the authors have any conflict of interest. This also certifies that ethical approval was sought from the competent authority and consent was obtained from all the patients before they underwent surgical debridement.
Ana Alastruey-Izquierdo has a research contract from the Spanish Network for the Research into Infectious Diseases (REIPI RD06/0008). No other author had any sort of grant from any source for conducting this study.