Scedosporium species and Lomentospora prolificans (Sc/Lp) are emerging molds that cause invasive disease associated with a high mortality rate. After Aspergillus, these molds are the second filamentous fungi recovered in lung transplant (LT) recipients.
AimsOur objective was to evaluate the incidence, risk factors and outcome of Sc/Lp infections in LT recipients at a tertiary care hospital with a national reference LT program.
MethodsA nine-year retrospective study was conducted.
ResultsDuring this period, 395 LT were performed. Positive cultures for Sc/Lp were obtained from twenty-one LT recipients. Twelve patients (incidence 3.04%) developed invasive scedosporiosis (IS). In 66.7% of the patients with IS the invasive infection was defined as a breakthrough one. The main sites of infection were lungs and paranasal sinuses. Most of the patients received combination antifungal therapy. The IS crude mortality rate after 30 days was 16.7%, and 33.3% after a year.
ConclusionsOur study highlights improved survival rates associated with combination antifungal therapy in LT recipients and underlines the risk of breakthrough infections in patients with allograft dysfunction on nebulized lipidic amphotericin B prophylaxis. In addition to pretransplant colonization, acute or chronic organ dysfunctions seem to be the main risk factors for IS.
Las especies de Scedosporium y Lomentospora prolificans (Sc/Lp) son mohos emergentes que causan infecciones invasivas con una alta tasa de mortalidad. Después de Aspergillus, estos hongos filamentosos son los más frecuentemente aislados en pacientes receptores de trasplante de pulmón (TP).
ObjetivosNuestro objetivo fue evaluar la incidencia, los factores de riesgo y la evolución de la infección por Sc/Lp en receptores de TP en un hospital terciario de referencia nacional para TP.
MétodosSe realizó un estudio retrospectivo de nueve años.
ResultadosDurante este período se realizaron 395 TP. Veintiún receptores de TP tuvieron cultivos positivos para Sc/Lp, y doce de ellos desarrollaron escedosporiasis invasiva (SI) (incidencia del 3,04%). Se observaron infecciones de brecha en el 66,7% de los pacientes con SI. Los principales focos de infección fueron el pulmón y los senos paranasales. La mayoría de los pacientes recibieron terapia antifúngica combinada. La tasa de mortalidad bruta de la SI a los 30 días fue del 16,7%, ascendiendo al 33,3% al cabo de un año.
ConclusionesNuestro estudio destaca la mejora de la tasa de supervivencia asociada a la terapia antifúngica combinada en TP y subraya el riesgo de infecciones de brecha en pacientes con disfunción de injerto en profilaxis con anfotericina B lipídica nebulizada. La colonización previa al trasplante junto con la disfunción aguda o crónica del injerto parecen ser los principales factores de riesgo de la SI.
The incidence of invasive infections by Scedosporium apiospermum complex and Lomentospora prolificans (Sc/Lp) in lung transplant recipients (LTR) is increasing.4,11 In fact, Sc/Lp are the most recovered molds in respiratory samples after Aspergillus in LTR. These fungi are a major concern due to their high intrinsic resistance to antifungal drugs4,10,13 and have been considered an absolute contraindication for transplantation in some programs.16,17 In addition, therapeutic approach has a wide variability among centers and the evidence used is largely based on case studies which have shown highly inconsistent results.14 Our aim was to evaluate the incidence, risk factors and outcome of Sc/Lp infection in LTR patients at La Fe University Hospital (Valencia, Spain), a tertiary care hospital with a national reference lung transplant (LT) program.
Clinical data of LTR from whom Sc/Lp was isolated during 2011–2019, were reviewed. Updated EORTC/MSG consensus definitions and International Society for Heart and Lung Transplantation (ISHLT) criteria were used to classify invasive scedosporiosis (IS) episodes as “proven”, “probable” or “possible”.7,12 Colonization was defined as positive respiratory samples in asymptomatic patients with absence of characteristics of endobronchial/lung lesions.1,9,11Sc/Lp species were isolated and identified using the standard morphological methodology.5 The antifungal susceptibility testing with a microdilution method (SensititreYeastOne, Thermo-Fisher, Madrid, Spain) was performed only on isolates causing therapeutic failure.
Thirty-day crude mortality and related mortality rates, as well as one-year evolution rate, were reviewed.
During the study period, a total of 395 LT were performed in our center. Standard immunotherapy consisted of oral cyclosporine (2.5mg/kg/12h), tacrolimus (0.15mg/kg/12h), intravenous mycophenolate mofetil (1500mg/12h during three weeks, and 1000mg/12h from then on) and methylprednisolone (0.5mg/kg/day and tapering down during 3 months to 10mg/day). Induction immunosuppression is not used in our center and, therefore, it was not used in these patients (i.e. anti-lymphocyte globulin or basiliximab). Acute cellular rejection (ACR) episodes associated with grade A2 or higher clinical symptoms were treated. In order to optimize immunosuppression maintenance, the first-line treatment of ACR consisted of intravenous methylprednisolone 10mg/kg daily for 3 days, followed by prednisone, starting at 0.5mg/kg/day and decreasing by 5mg every five days until the baseline dose. Antifungal prophylaxis in LTR in our center consists of nebulized lipidic amphotericin B. Antifungal preemptive therapy in LT patients previously colonized with Sc/Lp in our center includes any combination of triazoles (voriconazole or posaconazole) and terbinafine. Isavuconazole had not yet been approved when this study started.
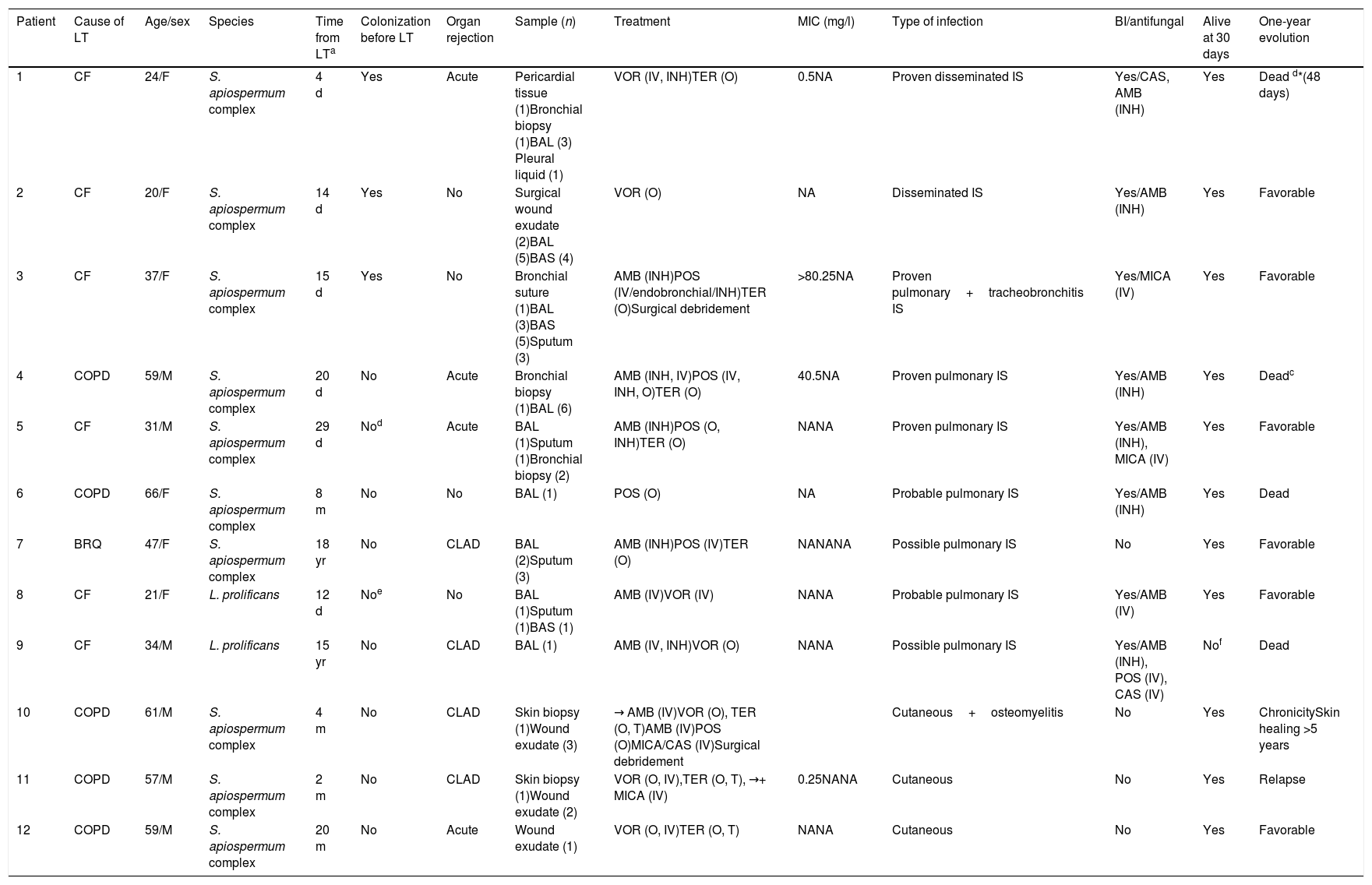
There were twelve invasive infections by Sc/Lp within this population (incidence 3.04%), and all of them underwent bilateral LT, except for patient #6. Among these episodes, seven affected the lungs, three involved skin and soft tissue, and two were disseminated infections. All of them were caused by Scedosporium apiospermum complex, except for two pulmonary infections which were caused by L. prolificans. Clinical features, treatment and outcome are summarized in Table 1.
Clinical characteristics of lung transplant recipients with scedosporiosis/lomentosporiosis.
| Patient | Cause of LT | Age/sex | Species | Time from LTa | Colonization before LT | Organ rejection | Sample (n) | Treatment | MIC (mg/l) | Type of infection | BI/antifungal | Alive at 30 days | One-year evolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CF | 24/F | S. apiospermum complex | 4 d | Yes | Acute | Pericardial tissue (1)Bronchial biopsy (1)BAL (3) Pleural liquid (1) | VOR (IV, INH)TER (O) | 0.5NA | Proven disseminated IS | Yes/CAS, AMB (INH) | Yes | Dead d*(48 days) |
| 2 | CF | 20/F | S. apiospermum complex | 14 d | Yes | No | Surgical wound exudate (2)BAL (5)BAS (4) | VOR (O) | NA | Disseminated IS | Yes/AMB (INH) | Yes | Favorable |
| 3 | CF | 37/F | S. apiospermum complex | 15 d | Yes | No | Bronchial suture (1)BAL (3)BAS (5)Sputum (3) | AMB (INH)POS (IV/endobronchial/INH)TER (O)Surgical debridement | >80.25NA | Proven pulmonary+tracheobronchitis IS | Yes/MICA (IV) | Yes | Favorable |
| 4 | COPD | 59/M | S. apiospermum complex | 20 d | No | Acute | Bronchial biopsy (1)BAL (6) | AMB (INH, IV)POS (IV, INH, O)TER (O) | 40.5NA | Proven pulmonary IS | Yes/AMB (INH) | Yes | Deadc |
| 5 | CF | 31/M | S. apiospermum complex | 29 d | Nod | Acute | BAL (1)Sputum (1)Bronchial biopsy (2) | AMB (INH)POS (O, INH)TER (O) | NANA | Proven pulmonary IS | Yes/AMB (INH), MICA (IV) | Yes | Favorable |
| 6 | COPD | 66/F | S. apiospermum complex | 8 m | No | No | BAL (1) | POS (O) | NA | Probable pulmonary IS | Yes/AMB (INH) | Yes | Dead |
| 7 | BRQ | 47/F | S. apiospermum complex | 18 yr | No | CLAD | BAL (2)Sputum (3) | AMB (INH)POS (IV)TER (O) | NANANA | Possible pulmonary IS | No | Yes | Favorable |
| 8 | CF | 21/F | L. prolificans | 12 d | Noe | No | BAL (1)Sputum (1)BAS (1) | AMB (IV)VOR (IV) | NANA | Probable pulmonary IS | Yes/AMB (IV) | Yes | Favorable |
| 9 | CF | 34/M | L. prolificans | 15 yr | No | CLAD | BAL (1) | AMB (IV, INH)VOR (O) | NANA | Possible pulmonary IS | Yes/AMB (INH), POS (IV), CAS (IV) | Nof | Dead |
| 10 | COPD | 61/M | S. apiospermum complex | 4 m | No | CLAD | Skin biopsy (1)Wound exudate (3) | → AMB (IV)VOR (O), TER (O, T)AMB (IV)POS (O)MICA/CAS (IV)Surgical debridement | Cutaneous+osteomyelitis | No | Yes | ChronicitySkin healing >5 years | |
| 11 | COPD | 57/M | S. apiospermum complex | 2 m | No | CLAD | Skin biopsy (1)Wound exudate (2) | VOR (O, IV),TER (O, T), →+ MICA (IV) | 0.25NANA | Cutaneous | No | Yes | Relapse |
| 12 | COPD | 59/M | S. apiospermum complex | 20 m | No | Acute | Wound exudate (1) | VOR (O, IV)TER (O, T) | NANA | Cutaneous | No | Yes | Favorable |
LT: lung transplantation; MIC: minimal inhibitory concentration; BI: breakthrough infection; COPD: chronic obstructive pulmonary disease; CF: cystic fibrosis; BRQ: bronchiectasis; M: male; F: female; d: days; m: months; yr: years; CLAD: chronic lung allograft dysfunction; BAL: bronchoalveolar lavage; BAS: bronchoaspirate; AMB: amphotericin B; POS: posaconazole; TER: terbinafine; VOR: voriconazole; MICA: micafungin; CAS: caspofungin; INH: inhaled therapy; IV: intravenous therapy; O: oral therapy; T: topic therapy; NA: not available; IS: invasive scedosporiosis; CMV: cytomegalovirus. → Arrows indicate changes in treatment.
Attributable dead with acute organ rejection, growth of S. apiospermum complex in bronchial biopsy and coinfection with CMV.
Chronic colonization with S. apiospermum complex three years before transplantation, with no isolates ever after.
Pretransplant colonization by Sc/Lp has been related to early infection (first month post-transplantation) with poor outcome, despite antifungal prophylaxis.8,11 There were eight patients colonized by Sc/Lp before transplantation, three of them developed IS in the early post-operative period (less than 15 days after surgery), and one of them (#1) died after a thoracic dissemination in the context of increased immunosuppression due to acute rejection. Based on our experience, aggressive pretransplant antifungal prophylaxis with a triazole and intensive preemptive antifungal therapy after surgery are crucial to having a good outcome and should be implemented in pretransplant colonized receptors.
Interestingly, the isolation of Sc/Lp in the respiratory tract of LTR does not necessarily mean invasive disease in the late post-transplant period.17 In our study, 9 LTR in whom S. apiospermum complex (4 patients) or L. prolificans (5 patients) were isolated in respiratory samples (sputum, bronchoaspirate or bronchoalveolar lavage) never developed IS (42.8%). Sc/Lp were found from 3 to 50 months after LT, and only two of these patients received a preemptive antifungal treatment. Thus, microbiological findings should be interpreted always in conjunction with patient risk factors, clinical signs and imaging techniques.
There were eight infections in patients suffering either chronic lung allograft dysfunction (4 patients), or acute rejection (4 patients); interestingly, three out of four late infections (more than six months after LT had passed), occurred during chronic (2/4), or acute rejection episodes (1/4). Intensification of immunosuppression was the main risk factor for the fungal infection, as shown in a previous study.8 Cutaneous infection by Sc/Lp usually occurs by traumatic inoculation or by hematogenous dissemination. In our study, the three cutaneous infections found were caused by S. apiospermum complex more than two months after transplantation. These patients were receiving intensive immunosuppressive therapy due to graft rejection episodes. All of them recovered very slowly despite prolonged antifungal treatment (from six months to years of treatment), possibly because the initial focus could not be controlled or a more extensive surgical debridement could not be performed. In absence of previous trauma, a hematogenous dissemination from an undetected respiratory tract colonization was considered the origin of these three episodes.
Breakthrough infections were found in 8 of 12 patients (66.7%), six by S. apiospermum complex and two pulmonary infections by L. prolificans; all but one LTR had received prophylaxis with inhaled amphotericin B lipid complex. This is very similar to what is described in the literature, where 67–100% of IS in LTR are breakthrough infections.2,15,19
Although L. prolificans has been associated with sepsis and positive blood cultures in neutropenic patients, we did not find this species in blood or causing disseminated infection. Therefore, neutropenia appears to have more impact on the incidence and severity of disseminated Sc/Lp infections than solid organ transplant immunosuppression regimens.6
The Sc/Lp infection was treated in all our patients, except for patients #2 and #6, with combined antifungal therapy, being the most frequent combination liposomal amphotericin B plus a triazole (with or without terbinafine). Furthermore, in patients #1, #3, #4 and #5, aerosolized and/or endobronchial instillation of posaconazole was added to the conventional treatment, as we have reported before.18
One year after the Sc/Lp infection 4 of the 12 patients (33.3%) had died (Table 1). In two of these four patients (#1 and #4) the death was related to the fungal infection, both by S. apiospermum complex. Patient #1 died after developing a disseminated infection on the 48th day of the immediate postoperative period in spite of salvage therapy administered with posaconazole local instillations. During the first month post-diagnosis only one patient died (#9), but her dead was not related to IS.
In most studies, the Sc/Lp crude mortality rate reported is very high, ranging 30–70%, even 100% for L. prolificans; mortality is usually associated to dissemination, central nervous system infection, fungemia, and lack of recovery of neutrophils.4,8,10 To our knowledge, the global mortality observed in our study (33.3%) is one of the lowest ever reported. This low mortality could be explained by the lack of central nervous system infections, the few L. prolificans infections and the use of the combination of triazoles (systemic and local) and lipidic amphotericin B (nebulized or intravenous) in an attempt to reproduce the synergy reported in vitro.3 Based on this experience, combination therapy including voriconazole plus lipidic amphotericin B, terbinafine, and local therapy with triazoles should be considered to treat refractory cases of tracheobronquial or lung infections by Sc/Lp species, although this proposal must be confirmed in further studies.
The present study had some limitations. The observational and retrospective design, together with the small sample size, did not allow to identify the risk factors for Sc/Lp infection nor the clinical aspects associated to a favorable outcome. Furthermore, the morphological identification of the species could not be confirmed by molecular techniques since most of the isolates were not stored.
Despite the limitations of the study, our case series is the largest invasive Sc/Lp infection in LTR case series ever published in Europe, and we present the lowest attributable mortality ever reported. This low mortality could be related to the aggressive antifungal therapy used post-lung transplant as preemptive and targeted therapy. The incidence of IS in LTR in our setting is 3.04%, with an increase in the risk of breakthrough infections for patients with allograft dysfunction and prolonged prophylaxis with nebulized lipidic amphotericin B. Finally, based on our experience, we highly recommend the implementation of combined antifungal therapy in IS as well as regular monitorization in Sc/Lp colonized LTR with acute or chronic lung dysfunction.