Trichosporon asahii, an emerging fungal pathogen, has been frequently associated with invasive infections in critically ill patients.
Case reportA 74-year-old male patient diagnosed with COVID-19 was admitted to an Intensive Care Unit (ICU). During hospitalization, the patient displayed episodes of bacteremia by Staphylococcus haemolyticus and a possible urinary tract infection by T. asahii. While the bacterial infection was successfully treated using broad-spectrum antibiotics, the fungal infection in the urinary tract was unsuccessfully treated with anidulafungin and persisted until the patient died.
ConclusionsWith the evolving COVID-19 pandemic, invasive fungal infections have been increasingly reported, mainly after taking immunosuppressant drugs associated with long-term broad-spectrum antibiotic therapy. Although Candida and Aspergillus are still the most prevalent invasive fungi, T. asahii and other agents have emerged in critically ill patients. Therefore, a proper surveillance and diagnosing any fungal infection are paramount, particularly in COVID-19 immunocompromised populations.
Trichosporon asahii, un hongo patógeno emergente, se ha asociado con frecuencia con infecciones invasivas en pacientes enfermos en estado crítico.
Caso clínicoUn paciente de sexo masculino de 74 años de edad, con diagnóstico positivo para la COVID-19, ingresó en una unidad de cuidados intensivos. Durante la hospitalización el paciente presentó episodios de bacteriemia por Staphylococcus haemolyticus y una posible infección del tracto urinario por T. asahii. Mientras la infección bacteriana fue tratada exitosamente con antibióticos de amplio espectro, la infección micótica urinaria no remitió con anidulafungina y persistió hasta la muerte del paciente.
ConclusionesCon la pandemia de la COVID-19 se han notificado cada vez más casos de infecciones micóticas invasivas, principalmente después del uso de fármacos inmunosupresores, asociados con terapia de antibióticos de amplio espectro. Aunque Candida y Aspergillus siguen siendo los hongos invasores más prevalentes, T.asahii y otras especies han emergido en pacientes enfermos en estado crítico. Por lo tanto, la vigilancia y el diagnóstico de las infecciones micóticas es primordial, particularmente en poblaciones inmunodeficientes por la COVID-19.
The COVID-19 pandemic has caused millions of deaths worldwide. It has been reported that patients with comorbidities, coinfections, and secondary infections have the worst prognosis.8,9 Noteworthy, Trichosporon asahii, an emerging pathogen associated with invasive infections in critically ill patients, has been an etiological agent of coinfection and fungemia in COVID-19 patients.1,2 In the present case study, bacteremia by Staphylococcus haemolyticus and a possible urinary tract infection (UTI) by T. asahii in a critically ill patient with COVID-19 is reported.
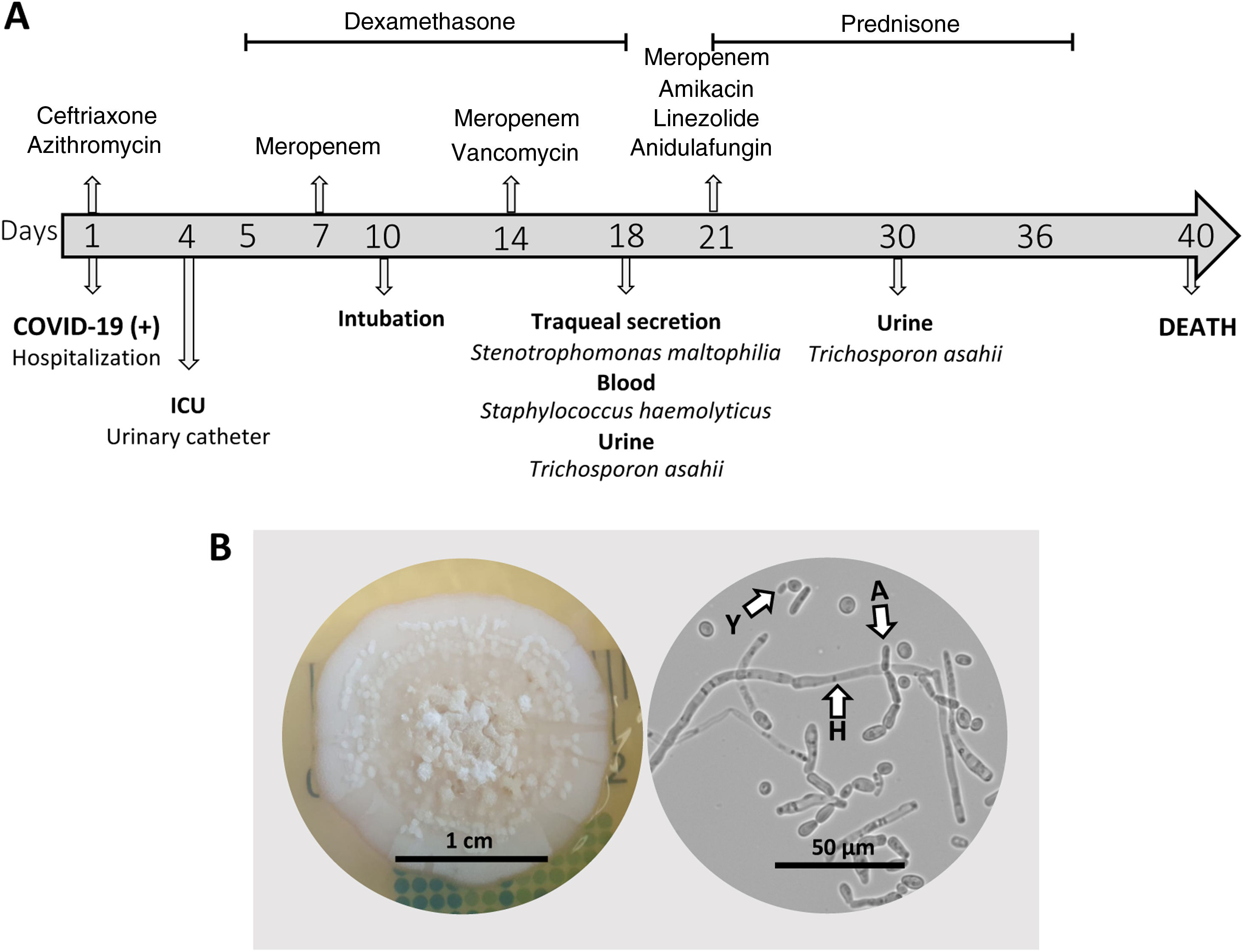
In January 2021, a 74-year-old male patient with a history of hypertension and type II diabetes was admitted to a Brazilian hospital with laboratory-confirmed SARS-CoV-2. For six days, the patient was experiencing diarrhea without mucus and blood (∼6 episodes/day), sporadic unproductive cough, asthenia, and unmeasured fever. Upon hospital admission, empirical antibacterial therapy with ceftriaxone (1g/24h, IV) and azithromycin (500mg/24h, PO) was administered (Fig. 1A).
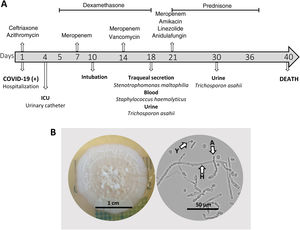
Clinical course and mycological data of a critically ill COVID-19 patient with a possible urinary tract infection caused by Trichosporon asahii. A: Timeline of the clinical evolution of the patient. B: Colony and microscopy of T. asahii culture on Sabouraud dextrose agar at 35°C for three days (Y: yeast, H: hyphae, and A: arthroconidia).
After three days, the patient presented hyponatremia (Na=126mEq/L), abnormal results of kidney function (114mg/dL urea, 2.5mg/dL creatinine), anemia (11.7g/dL hemoglobin), increased lactate dehydrogenase activity (366U/L) and higher concentration of C-reactive protein (PCR, 53mg/L), with normal leukocyte counts. Moreover, the urinalysis revealed the presence of hemoglobin and protein. The observed hematuria, proteinuria and renal insufficiency pointed to a nephritic syndrome. The patient was fitted with an indwelling urinary catheter and transferred to the COVID-19 intensive care unit (ICU).
On day 5, corticosteroid therapy with dexamethasone (6–10mg/24h) was started. This treatment lasted for eight days and was then switched to prednisone (40mg/24h) for 15 days. On day 7, antibiotic therapy was changed to meropenem (1g/12h, IV) and, after 7 days, vancomycin (1g/48h, IV) was added. On day 10, the patient underwent endotracheal intubation and mechanical ventilation (Fig. 1A).
Blood, tracheal secretion, and urine cultures were performed with samples collected 15 days after ICU admission (Fig. 1A). Bacterial and fungal species were identified using Vitek® 2 Compact (bioMérieux, São Paulo, Brazil) and confirmed by MALDI-TOF (Biotyper®, Bruker, Massachusetts, USA). S. haemolyticus was isolated from blood and exhibited clindamycin, erythromycin, gentamicin, levofloxacin, oxacillin, and rifampicin resistance; Stenotrophomonas maltophilia, susceptible to trimethoprim/sulfamethoxazole, was isolated from the tracheal secretion (>100,000CFU/mL). Interestingly, T. asahii (>100,000CFU/mL) was isolated on cystine lactose electrolyte deficient (CLED) agar from the urine sample. Despite the fungal presence, the patient did not exhibit any symptom related to an urinary tract infection. T. asahii isolate (which was assigned the reference HGN1) was subcultured on Sabouraud dextrose agar, and images of the colony and microscopic structures were captured (Fig. 1B).
On day 21, the patient was administered erythropoietin (4000UI, 3×/week) and a combined antimicrobial therapy comprised of meropenem (1g/12h, IV), amikacin (500mg/24h, IV), linezolid (600mg/12h, IV), and anidulafungin (100mg/12h, IV) (Fig. 1A). On day 29, a RT-PCR test showed the persistence of SARS-CoV-2 infection. Although new tracheal secretion and blood cultures were negative, T. asahii (>100,000CFU/mL) was still detected in the urine culture, which was associated with an increased number of leukocytes (>50 cells per field) and red blood cells (>30 cells per field) in the urine sediment. At that point the patient still had the indwelling urinary catheter, and there were no reports of replacement in the medical record. The patient died 40 days after hospital admission due to cardiac arrest and acute respiratory distress syndrome, being still SARS-CoV-2 positive (Fig. 1A).
The antifungal susceptibility testing performed using the EUCAST broth microdilution methodology (www.eucast.org) revealed that the T. asahii HGN1 strain was susceptible to amphotericin B (AMB, MIC=2μg/mL) and all tested azoles. The MICs obtained for fluconazole, voriconazole, itraconazole, posaconazole, and isavuconazole ranged between 0.03 and 1μg/mL. On the other hand, the MIC value of anidulafungin was quite high (>16μg/mL). It is well-known that AMB and echinocandins display poor in vitro activity against Trichosporon and echinocandins do not achieve therapeutic concentrations in the urine.4,5 It should also be pointed out that, among triazoles, the lowest MIC value in our study was that from voriconazole, considered the best choice to treat T. asahii infections.5T. asahii strain HGN1 was a medium biofilm former, a characterization made with the crystal violet staining method.7 Indeed, biofilm formation on medical devices, such as urinary catheters, is a cause of persistent infection, often associated with reduced antifungal susceptibility and increased virulence.3,7 Thus, it is likely that inadequate antifungal treatment with anidulafungin and the indwelling urinary catheter contributed to the observed T. asahii persistence in the urine.
In summary, we report a case of a possible UTI caused by the emerging and highly resistant pathogen T. asahii in a critically ill COVID-19 patient. Fungal infections have been increasingly reported during the evolving COVID-19 pandemic. Many cases have been associated with immunosuppressant drugs that treat immunological storms, as well as with long-term broad-spectrum antibiotic therapy and invasive medical devices. Thus, this study shows that while Candida and Aspergillus are still the most prevalent invasive fungi,10T. asahii and other agents are becoming more prevalent in critically ill patients.1,2,6 In this sense, surveillance and diagnosis are essential in preventing and treating fungal infections, especially in immunocompromised populations.
Ethical approvalAll data were gathered as part of routine work at the Hospital de Guarnição de Natal (Rio Grande do Norte State, Brazil). The Hospital de Guarnição de Natal ethical committee approved this study protocol (N27/2021).
FundingThis study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (2021/01279-5).
Conflict of interestNone declared.
DFFJ was a fellow from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance Code 001). KI and NL are research fellows of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (303373/2019-9 and 314336/2021-4, respectively).