Pulmonary mycoses resemble clinically and radiologically chronic pulmonary tuberculosis. Studies describing the prevalence, etiology and clinical features of pulmonary mycosis are of crucial importance in the Brazilian Amazon.
AimsTo estimate the frequency of pulmonary mycoses in smear-negative tuberculosis patients; to describe their demographic, epidemiological, and clinical characteristics; and to evaluate diagnostic methods.
MethodsA cross-sectional study was conducted at two tuberculosis reference institutions in Amazonas, Brazil. We included 213 patients and collected clinical data, blood and induced sputum to perform serological, direct microscopy, microbiologic culture and PCR-based assays to identify infections caused by Aspergillus fumigatus, Paracoccidioides brasiliensis, Histoplasma capsulatum, Cryptococcus, and HIV. Chest computed tomography was also performed.
ResultsPulmonary mycoses were diagnosed in 7% (15/213) of the cases, comprising ten aspergillosis cases, three cases of paracoccidioidomycosis and one case each of histoplasmosis and cryptococcosis. Among the patients with pulmonary mycoses, 86.7% were former tuberculosis patients. The most significant clinical characteristics associated with pulmonary mycoses were cavity-shaped lung injuries, prolonged chronic cough and hemoptysis.
ConclusionsOur study confirmed the high prevalence of pulmonary mycoses in smear-negative tuberculosis patients in the Brazilian Amazon.
Las micosis pulmonares se asemejan clínica y radiológicamente a la tuberculosis (TBC) pulmonar crónica. En la Amazonia brasileña se necesitan estudios que describan la prevalencia, la etiología y los factores clínicos de las micosis pulmonares.
ObjetivosEstimar la frecuencia de micosis pulmonares en pacientes con resultados negativos de baciloscopia para TBC, describir sus características demográficas, epidemiológicas y clínicas, y evaluar los métodos de diagnóstico.
MétodosSe realizó un estudio transversal en dos instituciones de referencia para el tratamiento de la TBC en el Estado de Amazonas, Brasil. Se incluyeron 213 pacientes y se recopilaron sus datos clínicos. Se tomaron muestras de sangre para estudios serológicos, de esputo inducido para examen microscópico directo y cultivos microbiológicos, y para efectuar PCR para Aspergillus fumigatus, Paracoccidioides brasiliensis, Histoplasma capsulatum y Cryptococcus. Además, se realizó tomografía axial computarizada.
ResultadosSe diagnosticaron micosis pulmonares en el 7% (15/213) de los casos. De ellos, 10 correspondieron a pacientes con aspergilosis (66,6%), tres a paracoccidioidomicosis (20%), uno a histoplasmosis (6,7%) y otro a criptococosis (6,7%). En este grupo, el 86,7% de los pacientes tenían antecedentes de TBC. Las características clínicas más significativas en estos enfermos con micosis pulmonares fueron la presencia de lesiones pulmonares cavitarias, la tos crónica y prolongada y la hemoptisis.
ConclusionesEste estudio muestra una elevada prevalencia de micosis pulmonares en pacientes con baciloscopia negativa para la TBC en la Amazonia brasileña.
Endemic mycoses are an important public health issue. They comprise the third most relevant group of neglected infectious diseases selected as a research priority in Latin America and the Caribbean.8 Tuberculosis (TB) has a high prevalence in these areas, and the number of cases of pulmonary mycoses that are found as pulmonary TB sequelae is increasing.6 The clinical and radiological similarities between pulmonary mycoses and TB may lead to a late diagnosis of the pulmonary mycosis and to administer empirical treatment for TB prior to the diagnosis of the underlying mycoses.12 In the Amazon region, smear-negative TB patients (SNTB) account for 35% of cases of pulmonary TB, summing up to almost twice of the recommended limit of 20% as suggested by the Health Ministry of Brazil (data source: National System for Surveillance and Control of Diseases). This situation highlights the importance of research dealing with pulmonary mycosis in patients with suspected SNTB.
Frequent mycoses with pulmonary manifestations in this population in the Brazilian Amazon comprise aspergillosis, paracoccidioidomycosis, cryptococcosis and histoplasmosis.2 Differential diagnosis of the pulmonary mycoses mentioned above is challenging, particularly if diagnostic options are scarce. Direct microscopy, microbiologic culture results, histopathology and serology applying agar gel-based double immunodiffusion testing have been the basis of the diagnosis of fungal infections for many decades in Brazil.15 However, PCR-based molecular diagnosis may increase both sensitivity and rapid diagnosis of fungal diseases.21 In the Brazilian Amazon, studies evaluating such diagnostic tools in endemic tropical areas are of ongoing importance in order to determine the best optimal locally applicable laboratory strategy.
Given this situation, the study was conducted to investigate the frequency of pulmonary mycoses in SNTB patients in the Brazilian Amazon, to determine the demographic, epidemiological, and clinical characteristics of patients with identified mycoses, and to comparably evaluate diagnostic approaches applied for the detection of pulmonary mycoses.
Materials and methodsEthical statementThis study was approved by the Tropical Medicine Foundation Dr. Heitor Vieira Dourado (FMT-HVD) ethical research committee (approval No 2081 on 12/16/2011). Informed consent was systematically obtained. All participants in the study specifically signed a voluntary consent form after being fully informed, and all participants were adults. Patient materials and forms were encoded to preserve anonymity and de-identified prior to analysis.
Study settingThis study was carried out at two reference centers, being the first for infectious respiratory diseases (Cardoso Fontes Polyclinic) and the second for infectious diseases in general (Tropical Medicine Foundation Dr. Heitor Vieira Dourado FMT-HVD). These centers are located in Manaus, the capital city of Amazonas State, with a population of approximately two million people. Amazonas State has the highest incidence of TB in Brazil, with 70% of its TB cases concentrated in Manaus city. Among these cases, 35% have lacked bacteriological confirmation (data source: National System for Surveillance and Control of Diseases [SINAN]).
Study designThis cross-sectional study was conducted from December 2012 to November 2013 and involved SNTB patients of both genders who were older than 18 years and had a history of cough, had provided two sputum samples that were smear negative (i.e., did not contain microscopically detectable acid-fast bacilli - AFB), and showed pulmonary radiological abnormalities.
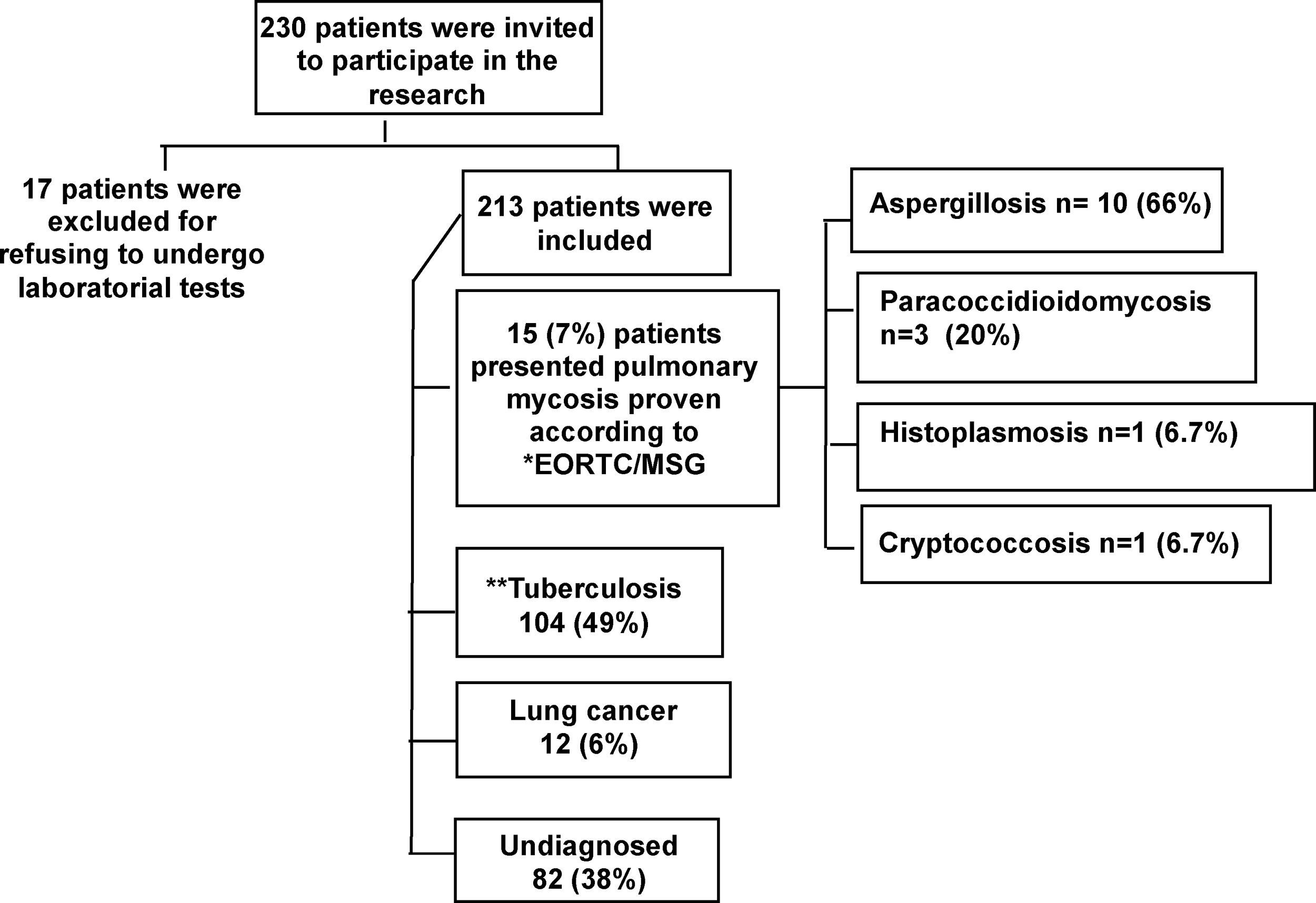
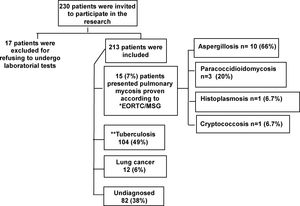
Information collectionA flow diagram of the data collection and study participant selection processes, including the distribution of a structured questionnaire about demographic information, medical and behavioral antecedents and clinical symptoms, is provided (Fig. 1).
Sample size calculationIn 2012, 445 SNTB patients attended both reference institutions. We calculated the sample size based on a 7% prevalence of fungal infections,11 a 4% margin of error and a confidence level of 95%. This calculation, using the software program Epi Info version 3.5.2 (CDC, Atlanta, GA, USA), resulted in 171 patients.
Data analysisThe data were analyzed using the statistics software R 3.0.1 (Free Software Foundation's GNU General Public License, Austria). Continuous numeric variables are reported as means (with standard deviations [SDs]) or medians (with interquartile ranges [IQRs]). Categorical variables are presented as the percentage of the total number of evaluated subjects. Applied statistical analyses comprised the chi-square test, Fisher's exact test, the t test and the Mann–Whitney test at a significance threshold of 5%. Association was calculated using the prevalence ratio, a method that has been previously applied in cross-sectional epidemiological studies.22,29
Sample collectionAfter the identification of suspected TB patients who had two negative smears and alterations in their chest x-ray, the selected participants were scheduled for sputum induction by inhalation of nebulized hypertonic saline solution (5 ml; 3% NaCl). Nebulization was performed using the ultrasonic nebulizer Inalasonic®. Adherence to appropriate biosafety norms was ensured. To reduce contamination, each patient brushed their teeth and the oral cavity prior to the collection of induced sputum samples. The induced sputum sample was submitted for TB detection by cultural growth, as well as for fungal detection by direct microscopy, cultural growth and fungus-specific PCR assays.
Culture-based TB study was performed using solid Ogawa-Kudoh egg-based medium.25 For the microscopic fungal study, samples clarified with 10% KOH and stained with Lactophenol Cotton Blue Stain and Naquin ink (produced by Faber Castell, São Carlos City, Brazil) were used. Fungal cultures were performed on Sabouraud dextrose agar and brain heart infusion agar from HiMedia Laboratories Pvt. Ltd. (Mumbai-India), and niger seed agar (Guizotia abyssinica 50g/l, glucose 1g/l, KH2PO4 1g/l, creatinine 1g/l, agar 15g/l, penicillin G 40 units/l and gentamicin 80mg/l). These microbiologic culture reagents were purchased from Difco, distributed by Interlab (São Paulo City, Brazil); all fungal cultures were incubated at room temperature (25 °C) or in an incubator at 37 °C for a period of 4–6 weeks. All culture approaches were performed under bio-safety level 3 laboratory conditions, in accordance with international standards to avoid laboratory contamination.33
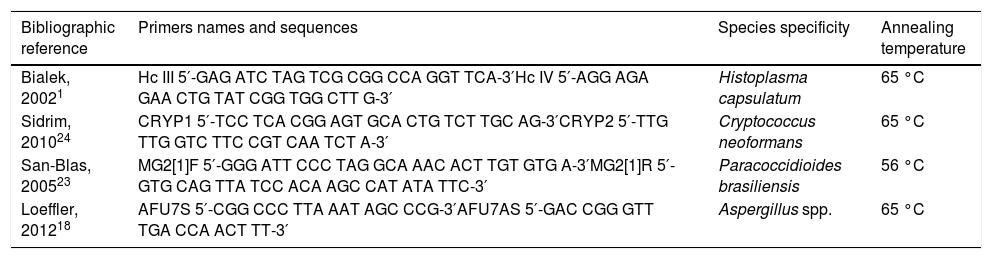
PCRDNA was extracted from the sputum samples using the PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA) following the recommendations of the manufacturer. The DNA within the samples was quantified by photometry at 260nm using a GeneQuant spectrophotometer (Eppendorf, Hamburg, Germany). In total, 20ng of the extracted DNA served as template for PCR amplification. The PCR reactions had a final volume of 25μl, containing PCR buffer (final concentration: 1×; 10mM Tris-HCl (pH 8.3) and 50mM KCl); 1.5mM MgCl2; 200nM primers, as listed in Table 1; 50 mM dNTPs; and 1U AmpliTaq DNA Polymerase (Life Technologies, São Paulo city, Brazil). The PCR was performed using a thermocycler (SuperCycle, Kyratec, Seoul, Republic of Korea) under the following conditions: initial denaturation of 5 min at 94 °C, 40 cycles of 30 s at 94 °C (denaturation), 30s at an annealing temperature according to Table 1, 90 s at 72 °C (extension), and a final extension step for about 10min at 72 °C. The PCR products were visualized by electrophoresis on a 2% agarose gel and stained with SYBR® Green (SYBR Safe DNA Gel Stain, Invitrogen, distributed by Life Technologies - São Paulo city, Brazil). A DNA Ladder Mix (SM0331, MBI Fermentas, St. Leon-Rot, Germany) served as a size marker (Table 1).
Primers used for the identification of pathogenic fungi and their specific annealing temperatures.
| Bibliographic reference | Primers names and sequences | Species specificity | Annealing temperature |
|---|---|---|---|
| Bialek, 20021 | Hc III 5′-GAG ATC TAG TCG CGG CCA GGT TCA-3′Hc IV 5′-AGG AGA GAA CTG TAT CGG TGG CTT G-3′ | Histoplasma capsulatum | 65 °C |
| Sidrim, 201024 | CRYP1 5′-TCC TCA CGG AGT GCA CTG TCT TGC AG-3′CRYP2 5′-TTG TTG GTC TTC CGT CAA TCT A-3′ | Cryptococcus neoformans | 65 °C |
| San-Blas, 200523 | MG2[1]F 5′-GGG ATT CCC TAG GCA AAC ACT TGT GTG A-3′MG2[1]R 5′-GTG CAG TTA TCC ACA AGC CAT ATA TTC-3′ | Paracoccidioides brasiliensis | 56 °C |
| Loeffler, 201218 | AFU7S 5′-CGG CCC TTA AAT AGC CCG-3′AFU7AS 5′-GAC CGG GTT TGA CCA ACT TT-3′ | Aspergillus spp. | 65 °C |
For serological assessments, 5 ml of blood were aseptically collected from a peripheral vein. Serology by agar gel-based double immunodiffusion testing was performed following an “in-house” protocol as described by Ouchterlony (1962).20 This test is based on the detection of specific antibodies against respective fungal agents via the formation of immune complexes that precipitate due to their high molecular weight, forming a visible line. The following antigens were used: (a) H. capsulatum (histoplasmin) culture filtrate antigen produced using the IGS 4/5 strain following the protocol described by Kaufman and Standard in 1976,27 (b) P. brasiliensis culture filtrate antigen produced using the Pb 339 strain following the protocol described by Fava-Netto,9 and (c) Aspergillus fumigatus culture filtrate antigen, which was produced using the JJG strain following the protocol described by Coleman and Kaufman in 1972.3
The Cryptococcal Antigen Latex Agglutination System (CALAS), distributed by Meridian Bioscience (Milan, Italy), was used to detect Cryptococcus antigens exactly as described by the manufacturer.
The RDTs (rapid diagnostic tests) Rapid Check HIV-1/2 and TR DPP HIV-1/2 SSP, distributed by Bio-Manguinhos/FIOCRUZ (Rio de Janeiro city, Brazil), were used to conduct anti-HIV antibody testing.
DefinitionsSmear-negative pulmonary TB (SNTB) was defined according to WHO guidelines: patients with at least two sputum samples negative for AFB, radiological abnormalities consistent with pulmonary TB and a decision by a physician to treat with a full course of TB chemotherapy.30 Cases of pulmonary mycoses were defined based on clinical, microbiologic and radiological criteria in accordance with the guidelines of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG).7 The present research followed the recommendations outlined in the “Strengthening of the Reporting of Observational Studies in Epidemiology” (STROBE) statement.31
ResultsThe prevalence of pulmonary mycosesThe prevalence of pulmonary mycoses in 213 SNTB patients was 7% (15/213). Pulmonary mycosis was defined in accordance with the EORTC/MSG guidelines.7 Aspergillosis and paracoccidioidomycosis were the most prevalent pulmonary mycoses (Fig. 2).
Flowchart showing the results of the present study. *European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG). **Patients who subsequently had a positive culture (56) or a negative or contaminated culture had a good clinical response to empirical treatment for TB.
Statistical analysis was conducted to investigate differences in the demographic and epidemiological characteristics of the groups with (n = 15) and without (n = 198) pulmonary mycoses (Table 2). The characteristics of the patients from both groups (n = 213) included the following: mean age of 51 years (SD: ±16), predominance of males, non-Caucasian ethnicity and living in the urban area of the city of Manaus. Two HIV-positive patients presented pulmonary mycoses. The statistical analysis showed that late morbidity characteristics such as hemoptysis (the prevalence ratio of this clinical condition was 3.76) and prolonged chronic cough were statistically significantly associated (p < 0.05) with pulmonary mycoses (Table 2).
Demographic, epidemiological and clinical characteristics of the 213 suspected SNTB patients with or without a diagnosis of pulmonary mycosis who were included in this cross-sectional study in Amazonas State, Brazil (2012–2013).
| Characteristic | Pulmonary mycosis | Totaln = 213 (%) | Prevalence ratio | aCI (95%) | bP value | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| n = 15 (%) | n = 198 (%) | |||||
| Age group (years) | ||||||
| > 31 | 14 (93.3) | 171 (86.4) | 185 (86.9) | 2.12 | 0.29–15.49 | 0.385 |
| = 30 | 1 (06.7) | 27 (13.6) | 28 (13.1) | |||
| Gender | ||||||
| Male | 11 (73.3) | 102 (51.5) | 113 (53.1) | 2.43 | 0.80–7.40 | 0.173 |
| Female | 4 (26.7) | 96 (48.5) | 100 (46.9) | 1.13 | ||
| Ethnicity | ||||||
| Caucasian | 05 (33.3) | 57 (28.8) | 62 (29.1) | 1.22 | 0.43–3.42 | 0.769 |
| Non-Caucasian | 10 (66.7) | 141 (71.2) | 151 (70.9) | |||
| Education | ||||||
| Illiterate | 4 (26.7) | 24 (12.1) | 28 (13.1) | 2.39 | 0.82–6.99 | 0.116 |
| ≥ 1 year | 11 (73.3) | 174 (87.9) | 185 (86.9) | |||
| Local housing | ||||||
| Rural | 03 (20) | 30 (15.2) | 33 (15.4) | 1.36 | 0.41–4.57 | 0.708 |
| Urban | 12 (80) | 168 (84.8) | 180 (84.6) | |||
| Previous TB | ||||||
| Yes | 13 (86.7) | 164 (83) | 177 (83) | 1.35 | 0.27–9.08 | 0.518 |
| No | 2 (13.3) | 34 (17) | 36 (17) | |||
| Smoking | ||||||
| Yes | 09 (60) | 114 (57.6) | 123 (57.7) | 1.94 | 0.71–5.24 | 0.292 |
| No | 6 (40) | 84 (42.4) | 90 (42.3) | |||
| Alcohol consumer | ||||||
| Yes | 7 (46.7) | 52 (26.3) | 59 (27.7) | 2.28 | 0.87–6.02 | 0.130 |
| No | 8 (53.3) | 146 (73.7) | 154 (72.3) | |||
| HIV | ||||||
| Yes | 2 (13.3) | 39 (20.2) | 41 (19.2) | 0.63 | 0.15–2.67 | 0.741 |
| No | 13 (86.7) | 159 (79.8) | 172 (80.8) | |||
| Average number of days with cough | ||||||
| cMedian (IQR) | 120 (90–180) | 60 (30–120) | 0.002 | |||
| Hemoptysis | ||||||
| Yes | 11 (73.3) | 79 (39.9) | 90 (42.3) | 3.76 | 1.24–11.42 | 0.024 |
| No | 4 (26.1) | 119 (60.1) | 123 (57.7) | |||
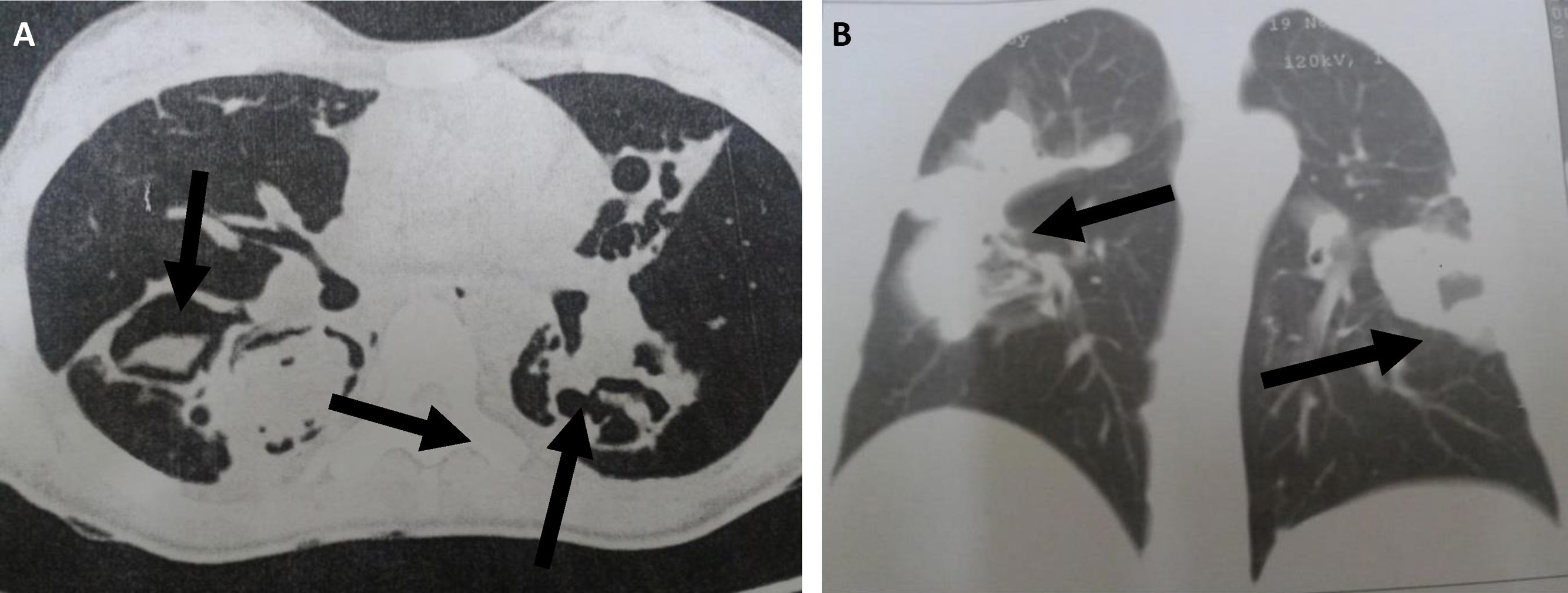
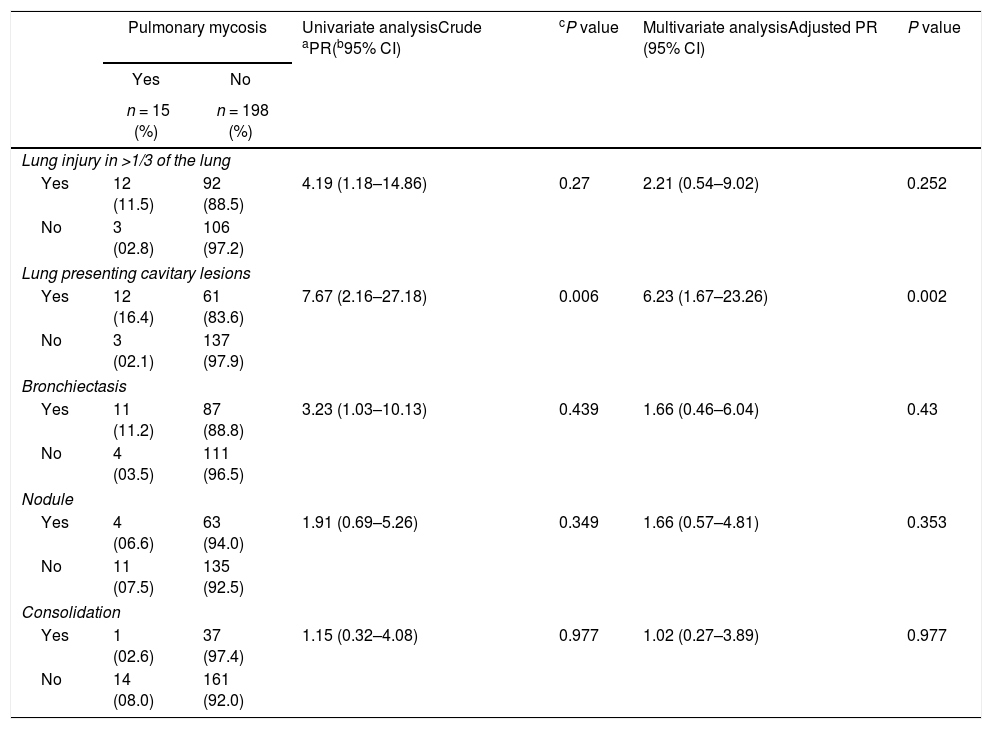
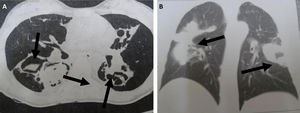
Table 3 shows the results for the main factors associated with the radiological findings of computed tomography (CT) in cases with or without pulmonary mycosis. In particular, the presentation of a cavitary lesion in the lung was associated with pulmonary mycosis. Representative examples of chest CT images from patients diagnosed with pulmonary mycoses are shown in Fig. 3.
Univariate and multivariate Poisson regression analyses of factors independently associated with pulmonary mycosis in patients included in this cross-sectional study in Amazonas State, Brazil (2012–2013).
| Pulmonary mycosis | Univariate analysisCrude aPR(b95% CI) | cP value | Multivariate analysisAdjusted PR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| n = 15 (%) | n = 198 (%) | |||||
| Lung injury in >1/3 of the lung | ||||||
| Yes | 12 (11.5) | 92 (88.5) | 4.19 (1.18–14.86) | 0.27 | 2.21 (0.54–9.02) | 0.252 |
| No | 3 (02.8) | 106 (97.2) | ||||
| Lung presenting cavitary lesions | ||||||
| Yes | 12 (16.4) | 61 (83.6) | 7.67 (2.16–27.18) | 0.006 | 6.23 (1.67–23.26) | 0.002 |
| No | 3 (02.1) | 137 (97.9) | ||||
| Bronchiectasis | ||||||
| Yes | 11 (11.2) | 87 (88.8) | 3.23 (1.03–10.13) | 0.439 | 1.66 (0.46–6.04) | 0.43 |
| No | 4 (03.5) | 111 (96.5) | ||||
| Nodule | ||||||
| Yes | 4 (06.6) | 63 (94.0) | 1.91 (0.69–5.26) | 0.349 | 1.66 (0.57–4.81) | 0.353 |
| No | 11 (07.5) | 135 (92.5) | ||||
| Consolidation | ||||||
| Yes | 1 (02.6) | 37 (97.4) | 1.15 (0.32–4.08) | 0.977 | 1.02 (0.27–3.89) | 0.977 |
| No | 14 (08.0) | 161 (92.0) | ||||
Chest CT scans of patients diagnosed with pulmonary mycoses. (A) Aspergillosis: CT section revealing bilateral lesions with cavities containing material with soft-tissue density and surrounding air, forming the air crescent sign (arrows); (B) Cryptococcosis: CT section showing bilateral parenchymal opacities with a pseudo-tumor aspect (arrows).
Table 4 shows the results of the laboratory analyses carried out for the diagnosis of pulmonary mycosis. Blood samples from all patients underwent serological tests for HIV and fungal infections. Additionally, sputum samples were studied by direct microscopy, culture and PCR. Serology was selected due to its better performance (accuracy) compared with other laboratory techniques, as demonstrated in previous studies.7
Laboratory results on 15 patients with a diagnosis of pulmonary mycosis who were included in this cross-sectional study in Amazonas State, Brazil (2012–2013).
| No of case | Diagnosis | Age(years) | Gender | HIV | Direct microscopic examination | Culture | Serology | PCRanalysis of sputum |
|---|---|---|---|---|---|---|---|---|
| 1 | Aspergillosis | 80 | F | − | − | − | Aspergillus fumigatus | Aspergillus spp. |
| 2 | Aspergillosis | 70 | M | − | − | Aspergillus fumigatus | Aspergillus fumigatus | Aspergillus spp. |
| 3 | Aspergillosis | 19 | F | − | − | − | Aspergillus fumigatus | Aspergillus spp. |
| 4 | Aspergillosis | 44 | M | − | − | Aspergillus fumigatus | Aspergillus fumigatus | − |
| 5 | Aspergillosis | 65 | M | − | − | Aspergillus fumigatus | Aspergillus fumigatus | Aspergillus spp. |
| 6 | Aspergillosis | 62 | M | − | − | Aspergillus fumigatus | Aspergillus fumigatus | Aspergillus spp. |
| 7 | Aspergillosis | 57 | F | − | − | Aspergillus fumigatus | Aspergillus fumigatus | Aspergillus spp. |
| 8 | Aspergillosis | 35 | M | − | aA | Aspergillus fumigatus | Aspergillus fumigatus | Aspergillus spp. |
| 9 | Aspergillosis | 77 | M | + | − | Aspergillus fumigatus | Aspergillus fumigatus | Aspergillus spp. |
| 10 | Aspergillosis | 37 | F | − | − | Aspergillus fumigatus | Aspergillus fumigatus | Aspergillus spp. |
| 11 | Paracoccidioidomycosis | 52 | M | − | bP | − | P. brasiliensis | − |
| 12 | Paracoccidioidomycosis | 54 | M | − | P | − | P. brasiliensis | − |
| 13 | Paracoccidioidomycosis | 43 | M | − | P | P. brasiliensis | P. brasiliensis | Paracoccidioides brasiliensis |
| 14 | Histoplasmosis | 42 | M | + | − | Histoplasma capsulatum | Histoplasma capsulatum | Histoplasma capsulatum |
| 15 | Cryptococcosis | 60 | M | − | cC | − | Cryptococcus spp. | Cryptococcus spp. |
Serology succeeded in diagnosing all 15 cases of pulmonary mycoses. Cultures of pulmonary secretions resulted in (a) seven isolates of Aspergillusflavus, obtained among the 10 samples collected from patients who presented with positive serology for aspergillosis; (b) one isolate of P. brasiliensis, confirming only one of three positive serology results for paracoccidioidomycosis; and (c) one H. capsulatum strain, isolated from the sputum sample of one HIV/AIDS patient. Microscopy showed poor correlation with other techniques, although this methodology was useful for the diagnosis of paracoccidioidomycosis and cryptococcosis cases, whereas the PCR confirmed most of the serological results.
DiscussionPulmonary mycoses, regardless of their association with TB, remain neglected in the Brazilian Amazon. In the Brazilian Amazon, several patients with pulmonary mycoses have been previously empirically treated as TB patients (unpublished data). These misdiagnoses most likely occurred due to the high TB prevalence in the region,32 the pronounced clinical and radiological similarities between chronic pulmonary mycoses and chronic TB,12 the lack of capacity of the healthcare system to address pulmonary mycoses, and an absence of reliable data on the prevalence, morbidity and mortality of pulmonary mycoses in the region.
The prevalence of pulmonary mycoses observed in the present study was similar to the prevalence found by Jacomelli et al. (2012). These authors evaluated the etiology of pulmonary infections among 286 SNTB patients referred to the Department of Pulmonology of the Faculty of Medicine, University of São Paulo, Brazil. These authors sent the patients for bronchoscopy and, when necessary, collected a transbronchial biopsy.11
Aspergillosis was the most prevalent pulmonary mycosis in the present study. The WHO estimates that the number of aspergillosis cases per year occurring as a sequela of pulmonary TB is 372,000 worldwide and 5663 in Brazil (incidence of 9.6/100,000).6 In the present work all ten cases of aspergillosis were diagnosed in patients who had previously suffered from tuberculosis. Amazonas State shows the highest incidence of TB in Brazil (70.6/100,000),22 and we estimate that 192 new aspergillosis cases emerge per year. Unfortunately, the vast majority of these patients is undiagnosed or is repeatedly empirically treated as TB patients.5
Paracoccidioidomycosis is of major medical interest in the countries of Latin America26 and, indeed, this mycosis was the second most prevalent pulmonary mycosis found in the present study. Even as the eighth leading cause of mortality among chronic infectious diseases in Brazil,4 paracoccidioidomycosis remains a neglected disease without research funding by government programs. In the present study, three cases of paracoccidioidomycosis were observed among 15 cases of detected pulmonary mycoses and among a total of 213 study participants.
In the current study, histoplasmosis was diagnosed in one patient with HIV/AIDS. This result was expected despite the results of previous studies that demonstrated through intradermal testing a high prevalence of contact with the agent (12.8–43.4%). Symptomatic cases of the disease are nearly always associated with AIDS, except for a few descriptions of small outbreaks and a few isolated cases.19,28
Cryptococcosis is a systemic mycosis frequently associated with HIV/AIDS. Regional studies suggest that 75% of diagnosed cryptococcosis cases affect HIV/AIDS patients.13,16 However, the patient in the present study was HIV negative. Cryptococcosis in HIV-negative patients is frequently caused by Cryptococcus gattii (genotype VGII).10 The present study suggests that the combination of serological and PCR-based assays might be useful for the detection and confirmation of pulmonary mycoses. In particular, PCR successfully identified most serologically identified cases of cryptococcosis, paracoccidioidomycosis, histoplasmosis and aspergillosis. However, the aspergillosis cases must be interpreted cautiously because a proof based on DNA alone cannot discriminate colonization from invasive disease. Additionally, the sensitivity of culture and PCR for the detection of Paracoccidioides was low compared with agar gel-based double immunodiffusion testing and direct microscopic examination. This result was previously shown in a study that suggested the low sensitivity of culture and the use of nested PCR instead of traditional PCR for the detection of P. brasiliensis in sputum.14 Future studies comprising highly sensitive real-time PCR protocols and alternative nucleic acid extraction strategies are therefore desirable. One of the most important results of the present work was proving the association of hemoptysis, cavitary lesions in the lung and chronic cough with pulmonary mycoses in the patients assessed. These results reinforce the necessity of sensitizing professionals working in TB control programs in the Brazilian Amazon regarding the need of correlating radiological findings, as described in this study, with pulmonary mycoses.
In this study, the immunodiagnostic assays did not include the detection of infections caused by the species Aspergillus niger, A. flavus or Paracoccidioides lutzii, which is a limitation. New studies should use or develop serological assays to evaluate the prevalence of the aforementioned causative agents of pulmonary mycoses. Serology results should also be carefully interpreted when obtained from immunocompromised patients, such as those with AIDS.17 Another limitation of the present study is the fact that traditional PCR, which is being increasingly abandoned in routine diagnosis in many industrialized Western countries, was used. Modern real-time PCR, e.g., hybridization probe-based (“LightCycler”) or hydrolysis probe-based (“TaqMan”) PCR, provides higher specificity due to the use of three or more DNA-binding elements instead of only two primers as applied in conventional PCR.
As a secondary finding in this study, TB was confirmed in 49% of the patients (104/213); those patients had been considered as SNTB at the beginning of the study, including 56 patients with positive culture for TB and those who had good clinical response to empirical TB treatment, and this diagnosis could be confirmed in the subsequent assessments. This finding is of importance for the public health policy in Northern Brazil.
Our study concludes that a clinically relevant prevalence of pulmonary mycoses exists among suspected SNTB patients in the Brazilian Amazon. Cavitary lesions in the lung, prolonged chronic cough and hemoptysis are frequent in these patients in case of pulmonary mycoses. Immunodiagnostic assays and sputum PCR were important tools for laboratorial diagnosis of pulmonary mycoses in the assessed settings.
This study was presented as part of the doctoral degree thesis of JSM at the Tropical Medicine Post-Graduate Program at Amazonas State University/Dr. Heitor Vieira Dourado Tropical Medicine Foundation.
Authors’ contributionsConceiving of the study and designing of the experiments: JSM, BW, JVBS, and FEME. JSM followed up and treated all the patients included.
Performance of the experiments: CSSS, DRT, RCSC, and JVBS. AASB, JSM, and FEME analyzed the data.
Contribution with reagents/materials/analysis tools: JSM, MMM, and JVBS.
JSM, BW, FH, JVBS, and FEME wrote the paper.
FundingThis work was supported by the “Innovative approaches for TB control in Brazil” grant, number 1 U2R TW006883-09, Program ICOHRTA AIDS/TB, sponsored by Fogarty International Center/National Institutes of Health, USA, and Fundação de Amparo á Pesquisa do Estado do Amazonas - FAPEAM Edict • call n. 001/2013-PPSUS, process: 062.00649/2014. The funding sources played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestsThe authors declare that there are not conflicts of interest.
We would like to thank Dr. Irineide Antunes Assumpção and all employees of the Cardoso Fontes Polyclinic/AM/Brazil for their cooperation and assistance in the execution of this study.