The emergence of resistant isolates has brought challenges to the treatment of sporotrichosis, prompting the search for new therapeutic strategies. Previous studies reported that nonsteroidal anti-inflammatory drugs (NSAIDs) show in vitro activity against several pathogenic fungi, including species of Candida, Cryptococcus, and Trichosporon.
AimsThis study aimed to investigate the in vitro efficacy of three NSAIDs (acetylsalicylic acid, diclofenac sodium, and ibuprofen), alone and in combination with itraconazole, against eleven clinical isolates of Sporothrix brasiliensis and Sporothrix schenckii.
MethodsMinimal inhibitory concentrations were determined by the broth microdilution method. Drug interactions and the fractional inhibitory concentration index of NSAIDs and itraconazole were assessed by the checkerboard method.
ResultsWhen used alone, ibuprofen was the most active NSAID, followed by acetylsalicylic acid. Combinations of NSAIDs with itraconazole showed synergistic antifungal activity against nine isolates. It was also found that itraconazole combined with acetylsalicylic acid, diclofenac sodium, or ibuprofen, led to resistance reversal in two, three, and five of the six drug-resistant isolates, respectively.
ConclusionsThe results indicate that the combination of itraconazole and the evaluated NSAIDs are a promising strategy for the treatment of sporotrichosis.
La aparición de cepas resistentes a los antifúngicos convencionales ha supuesto un nuevo desafío en el tratamiento de la esporotricosis y ha impulsado la búsqueda de nuevas estrategias terapéuticas. Estudios anteriores han descrito que los antiinflamatorios no esteroideos (AINE) tienen actividad in vitro contra diversos hongos patógenos, incluidas especies de Candida, Cryptococcus y Trichosporon.
ObjetivosEste estudio tuvo como objetivo investigar la eficacia in vitro de tres AINE (ácido acetilsalicílico, diclofenaco sódico e ibuprofeno), aisladamente y en combinación con itraconazol, contra once aislamientos clínicos de Sporothrix brasiliensis y Sporothrix schenckii.
MétodosLas concentraciones inhibitorias mínimas fueron determinadas según el método de microdilución en caldo. Las interacciones farmacológicas y el índice de concentración inhibitoria fraccional de los AINE y de itraconazol fueran evaluadas mediante el método del tablero de ajedrez.
ResultadosComo molécula única, el ibuprofeno fue el AINE más activo, seguido del ácido acetilsalicílico. Las combinaciones de los AINE con itraconazol exhibieron actividades antifúngicas sinérgicas frente a nueve aislamientos. También se observó que la combinación de itraconazol con ácido acetilsalicílico, diclofenaco sódico o ibuprofeno provocó la reversión de la resistencia en dos, tres y cinco de los seis aislamientos resistentes a los fármacos, respectivamente.
ConclusionesEstos resultados indican que la combinación de itraconazol con los AINE evaluados representa una estrategia prometedora en el tratamiento de la esporotricosis.
Sporotrichosis is a subcutaneous mycosis caused by members of the genus Sporothrix. These dimorphic fungi have a worldwide distribution and are hyperendemic in Brazil, China, and South Africa.38,39 In the Rio de Janeiro metropolitan area, Brazil, sporotrichosis has been considered an epidemic zoonosis since the 1990s. In this region, the major mode of transmission to humans is through contact with infected cats.30 The Oswaldo Cruz Foundation diagnosed about 5000 cases of human sporotrichosis between 1998 and 2015.19 The state of Rio Grande do Sul has the second-highest number of cases in Brazil, with 83 cases of human sporotrichosis diagnosed from 2010 to 2016 in the southern region. The number of actual cases is likely even greater, as the disease is generally underreported and the occurrence of feline sporotrichosis is high.26Sporothrix brasiliensis and Sporothrix schenckii are the most virulent species and are commonly associated with human and animal infection in Brazil.9,28 The treatment for sporotrichosis usually consists of long-term administration of itraconazole, potassium iodide, terbinafine, or amphotericin B, depending on the severity and location of the lesions.6,21,27
Sporotrichosis has emerged as an important fungal infection and public health problem in the past decades not only because of its epidemic spread but also because of the increase in strains that are resistant to conventional treatments.2,11,22,33,35,39 Studies have shown a synergistic interaction between antifungal and nonsteroidal anti-inflammatory drugs (NSAIDs) against different fungal species resistant to antifungal drugs.13,16,25,32,37 However, the antifungal mechanism of NSAIDs has not yet been fully elucidated.14,24
Host cells, as well as fungi, are sources of prostaglandins and oxylipins. Prostaglandins, mainly prostaglandin E, play an important role in fungal survival and, probably, in the evasion of the host's immune response during infection,17,20,21,23 thereby contributing to the spread of fungi.21 Thus, the combination of conventional antifungals with NSAIDs may be an important therapeutic option for the treatment of sporotrichosis, especially when caused by highly virulent species. This study aimed to evaluate the in vitro effectiveness of NSAIDs, alone and in combination with itraconazole, against S. brasiliensis and S. schenckii.
Material and methodsMicroorganismsSix clinical isolates of S. brasiliensis and five clinical isolates of S. schenckii with different drug susceptibility profiles were kindly provided by collaborative researchers. The strains had been previously identified and were deposited at the fungal culture collection of the Laboratory of Microbiology of the Federal University of Pampa, Rio Grande do Sul, Brazil. The isolates were cultured on potato dextrose agar for 7 days at 25°C, and maintained at −80°C in yeast peptone dextrose medium (Sigma–Aldrich, Steinheim, Germany) containing glycerol (15% w/v).
DrugsThe antifungal agent itraconazole (ITZ; Janssen Pharmaceutica, Beerse, Belgium) and the NSAIDs acetylsalicylic acid (ASA), diclofenac sodium (DIC), and ibuprofen (IBU) (Sigma–Aldrich, Steinheim, Germany) were obtained commercially. Terbinafine (TRB) and amphotericin B (AMB) (Sigma Chemical Co., USA) were used for susceptibility testing. Drug stock solutions were prepared in dimethyl sulfoxide (Sigma–Aldrich, Steinheim, Germany), and serial dilutions were performed using RPMI 1640 medium (Sigma–Aldrich, Steinheim, Germany) buffered with 3-(N-morpholino)-propanesulfonic acid (Sigma–Aldrich, Steinheim, Germany) to obtain final concentrations of 0.06–32μg/mL for ITZ and AMB, 0.06–8mg/mL for NSAIDs, and 0.01–16μg/mL for TRB.
Determination of minimal inhibitory concentration (MIC) and fractional inhibitory concentration index (FICI)The MIC of NSAIDs and ITZ was determined by the broth microdilution method in 96-well plates, according to the Clinical and Laboratory Standards Institute protocol M38-A2.12 MIC values were determined visually after incubation of the plates at 30°C for 48 h by comparison with the growth control (well containing RPMI 1640 medium only). The MIC was defined as the lowest concentration of drug capable of completely inhibiting the fungal growth.12
Interaction effects between NSAIDs and ITZ were studied with the checkerboard method in 96-well microdilution plates.15 The FICI was calculated and interactions were categorized as synergistic (FICI≤0.5), antagonistic (FICI>4), or additive (0.5<FICI≤4).37 All experiments were performed in duplicate.
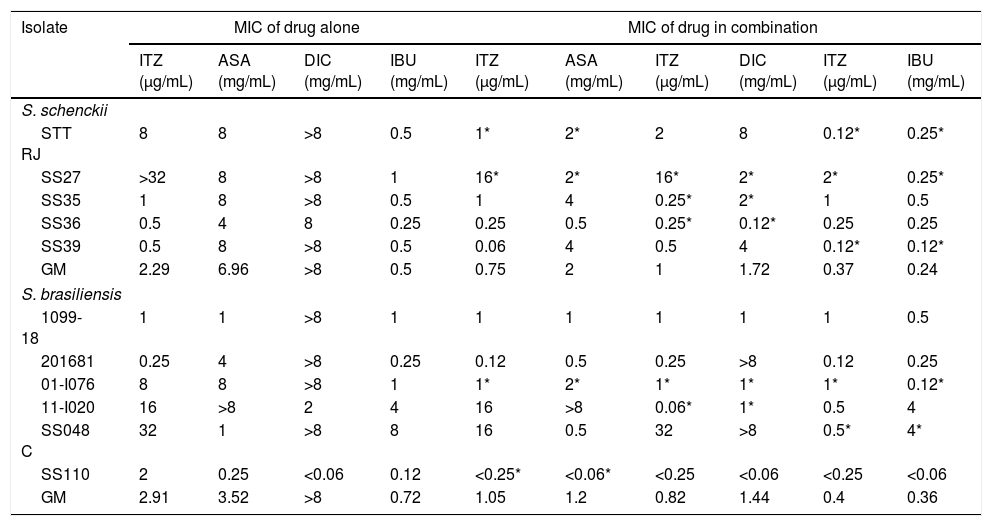
ResultsThe MIC values of ITZ, AMB, and TRB for S. schenckii isolates ranged from 0.5 to 32μg/mL, 0.5 to 2μg/mL, and 0.12 to 0.25μg/mL, respectively. Concerning S. brasiliensis isolates, ITZ, AMB, and TRB showed MIC values of 0.25–32μg/mL, 0.5–2μg/mL, and 0.01–0.5μg/mL, respectively. IBU and ASA showed some antifungal activity when tested alone, whereas DIC did not. The effects of NSAID and ITZ combinations are summarized in Table 1. The MIC of ITZ for nine of the isolates decreased when combining the drug with IBU (geometric mean decreased from 2.29 to 0.37μg/mL for S. schenckii and from 2.91 to 0.4 for S. brasiliensis). The interaction of ITZ with IBU was synergistic and inhibited five isolates (three isolates of S. schenckii and two of S. brasiliensis). Isolates for which the combination ITZ+IBU did not result in a lower MIC were sensitive to ITZ, with a MIC of 1μg/mL. The combination DIC+ITZ yielded a lower MIC for seven isolates. Synergistic effects were observed in three isolates of S. schenckii and two of S. brasiliensis with the combination ITZ+DIC. The combination of ITZ and ASA decreased MIC values for eight isolates, and synergistic effects were observed in two isolates of S. schenckii and two of S. brasiliensis. No antagonistic interactions between NSAIDs and ITZ were observed.
Minimal inhibitory concentrations (MIC) of itraconazole and nonsteroidal anti-inflammatory drugs, alone and in combination, against different isolates of Sporothrix schenckii sensu stricto and Sporothrix brasiliensis.
| Isolate | MIC of drug alone | MIC of drug in combination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ITZ (μg/mL) | ASA (mg/mL) | DIC (mg/mL) | IBU (mg/mL) | ITZ (μg/mL) | ASA (mg/mL) | ITZ (μg/mL) | DIC (mg/mL) | ITZ (μg/mL) | IBU (mg/mL) | |
| S. schenckii | ||||||||||
| STT RJ | 8 | 8 | >8 | 0.5 | 1* | 2* | 2 | 8 | 0.12* | 0.25* |
| SS27 | >32 | 8 | >8 | 1 | 16* | 2* | 16* | 2* | 2* | 0.25* |
| SS35 | 1 | 8 | >8 | 0.5 | 1 | 4 | 0.25* | 2* | 1 | 0.5 |
| SS36 | 0.5 | 4 | 8 | 0.25 | 0.25 | 0.5 | 0.25* | 0.12* | 0.25 | 0.25 |
| SS39 | 0.5 | 8 | >8 | 0.5 | 0.06 | 4 | 0.5 | 4 | 0.12* | 0.12* |
| GM | 2.29 | 6.96 | >8 | 0.5 | 0.75 | 2 | 1 | 1.72 | 0.37 | 0.24 |
| S. brasiliensis | ||||||||||
| 1099-18 | 1 | 1 | >8 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 |
| 201681 | 0.25 | 4 | >8 | 0.25 | 0.12 | 0.5 | 0.25 | >8 | 0.12 | 0.25 |
| 01-I076 | 8 | 8 | >8 | 1 | 1* | 2* | 1* | 1* | 1* | 0.12* |
| 11-I020 | 16 | >8 | 2 | 4 | 16 | >8 | 0.06* | 1* | 0.5 | 4 |
| SS048 C | 32 | 1 | >8 | 8 | 16 | 0.5 | 32 | >8 | 0.5* | 4* |
| SS110 | 2 | 0.25 | <0.06 | 0.12 | <0.25* | <0.06* | <0.25 | <0.06 | <0.25 | <0.06 |
| GM | 2.91 | 3.52 | >8 | 0.72 | 1.05 | 1.2 | 0.82 | 1.44 | 0.4 | 0.36 |
ITZ: itraconazole; ASA: acetylsalicylic acid; DIC: diclofenac sodium; IBU: ibuprofen; GM: geometric mean. An asterisk (*) indicates a synergistic effect (fractional inhibitory concentration index <0.5) between ITZ and the nonsteroidal anti-inflammatory drug.
The scarcity of antifungal drugs and the increase in drug-resistant fungal strains motivated this study on the activity of NSAIDs against isolates of Sporothrix spp. IBU and ASA alone showed low antifungal activity against the tested isolates, and DIC was the least active, with a median MIC greater than 8mg/mL for S. schenckii and S. brasiliensis. At lower concentrations (<1mg/mL), IBU inhibited the growth of 9 of the 11 isolates. It has been shown that IBU and ASA limit fungal growth1,9,29,37; however, to date, only one study has reported the inhibitory activity of IBU against Sporothrix.7 ITZ and NSAIDs showed synergistic activity against nine isolates. In S. brasiliensis, the synergistic effects were only observed in ITZ-resistant isolates, whereas in S. schenckii that synergism was also observed in ITZ-sensitive isolates.
In combination with antifungal agents, IBU and ASA showed antifungal activity against Trichosporon asahii,33,38Candida,13,26Cryptococcus,25Fusarium,34 and Pythium insidiosum.5 Rabbits infected with dermatophytes were completely cured after applying a cream containing 15mg/g IBU.4 Previous studies have shown that IBU had potent fungicidal activity at 10 mg/mL, causing damage to the fungal cell membrane by leading the leakage of intracellular potassium; at lower concentrations, IBU was shown to be fungistatic.25 In a study investigating the effects of IBU and ASA on the cell structure of Cryptococcusneoformans, the drugs drastically reduced the cell size and induced the production of reactive oxygen species, increasing oxidative stress and damaging the fungal membrane.24 IBU inhibited S. schenckii and S. brasiliensis growth at a median MIC of 256μg/mL.7 The referred study reported similar findings to those of the current study, in which S. schenckii was more susceptible to IBU combined with ITZ than S. brasiliensis. The same study showed that the combination of IBU and AMB induced reactive oxygen species accumulation and loss of plasma membrane integrity.7 In the present study, DIC showed no activity at the highest tested concentration (8mg/mL) against 8 of the 11 isolates. Regarding T. asahii, however, DIC was effective at concentrations of 1–4mg/mL.37 Interestingly, we observed synergism between DIC and ITZ in five isolates. Dupont et al. reported the cure of a mycetoma case using DIC once the surgery and the treatment with classical antifungals (voriconazole, posaconazole, and flucytosine) had failed.16
Studies have identified different modes of action of NSAIDs against fungi. Rusu et al.29 found that ASA and DIC may decrease the viability of Candida albicans by inhibiting cyclooxygenase isoenzymes, thereby suppressing the production of fungal prostaglandins. Likewise, the fungicidal activity of nimesulide against Trichophyton mentagrophytes and C. neoformans was shown to depend on a mechanism that inhibits fungal prostaglandins.14 Prostaglandins are produced by C. albicans and other pathogenic fungi, including S. schenckii,1,23 from both endogenous and exogenous sources of arachidonic acid. These compounds play an important role in fungal survival and, most likely, in the microorganism's capacity to evade the host's immune response.8 Prostaglandins induce germ tube production, hyphae formation, and fungal morphogenesis while suppressing the production of chemokines and tumor necrosis factor-alpha in the host.17,23 Noverr et al.23 and Tsitsigiannis et al.33 reported that Aspergillus fumigatus is capable of producing prostaglandins and cysteinyl leukotrienes from arachidonic acid. Furthermore, Tsitsigiannis et al.33 found that genes linked with the production of oxylipins in Aspergillus nidulans and A. fumigatus contribute to the production of fungal prostaglandins. Fungal oxylipin genes have a similar sequence to those of mammalian cyclooxygenases.33
As demonstrated by Wang et al.,36 NSAIDs may inhibit enzyme activity. The authors carried out an in vitro study and found that celecoxib disrupts the structure of Helicobacter pylori and inhibits urease activity, a crucial enzyme in the infection process. Urease is a nickel-dependent metalloenzyme that catalyzes the hydrolysis of urea to ammonia and carbon dioxide.10 Urease and proteases are important virulence factors of Sporothrix species, facilitating fungal colonization and tissue invasion. The expression of these enzymes is more significant in S. brasiliensis than in S. schenckii.2
Although the association of NSAIDs with ITZ does not always produce synergistic effects, the combined treatment leads to a reduction in ITZ MIC in many isolates; some isolates can be even reclassified as sensitive.3,18,31 In the present study, reversal of ITZ resistance was achieved with ITZ/ASA, ITZ/DIC, and ITZ/IBU treatments in two, three, and five of the six resistant isolates evaluated, respectively. The NSAIDs assessed in this study (ASA, DIC, and IBU) are commonly used to treat fever and other symptoms caused by sporotrichosis. Thus, the combination of NSAIDs and ITZ is a promising therapeutic alternative. However, it is difficult to predict the effect of NSAIDs in the treatment of Sporothrix infections, as treatment efficacy does not depend solely on antifungal activity. This study can guide future research on clinical combination therapies with NSAIDs and antifungal drugs against Sporothrix.
Conflict of interestThe authors declare that the research was carried out in the absence of any commercial or financial relationship that could be interpreted as a potential conflict of interest.