Cryptococcosis is a severe universally distributed mycosis which mainly affects immunocompromised hosts. This mycosis is caused by yeasts of two species complex of the genus Cryptococcus: Cryptococcus neoformans and Cryptococcus gattii. Meningeal cryptococcosis is the most frequent clinical presentation of this disseminated mycosis. The oral mucosa involvement is extremely unusual.
Case reportWe present a case of cryptococcosis with an unusual clinical form. The patient was assisted because she had an ulcerated lesion on the lingual mucosa. Encapsulated yeasts compatible with Cryptococcus were found in microscopic exams of wet preparations from lingual ulcer clinical samples obtained for cytodiagnosis and mycological studies. Cryptococcus neoformans (C. neoformans var. grubii VNI) was isolated in culture. This patient did not know her condition of HIV seropositive before the appearance of the tongue lesion.
ConclusionsThe involvement of the oral mucosa is uncommon in this fungal infection, but is important to include it in the differential diagnosis in HIV positive patients.
La criptococosis es una micosis grave de distribución universal que afecta principalmente a los huéspedes inmunodeficientes. Se han definido dos complejos de especies patógenas: Cryptococcus neoformans y Cryptococcus gattii. La meningoencefalitis es la presentación clínica más frecuente de esta micosis sistémica. La afectación de la mucosa oral es extremadamente rara.
Caso clínicoPresentamos el caso de una paciente VIH positiva con una forma clínica inusual de criptococosis. La enferma presentaba una lesión ulcerada en la punta de la lengua. El examen microscópico en fresco de la escarificación y de la biopsia de esta lesión mostraron levaduras capsuladas compatibles con Cryptococcus. Se obtuvo Cryptococcus neoformans (C. neoformans var. grubii VNI) en los cultivos. La paciente conoció su estado inmunológico (infección por VIH) en el contexto de esta enfermedad oportunista.
ConclusionesLa afectación de la mucosa oral es poco común en esta infección fúngica, pero es importante incluirla en el diagnóstico diferencial en pacientes VIH positivos.
Cryptococcosis is a severe universally distributed mycosis which mainly affects immunocompromised hosts. Some years ago, it was considered that the etiologic agent of this disease was a single species, Cryptococcus neoformans, which had two varieties: C. neoformans var. neoformans, and C. neoformans var. gattii. Currently, two species have been defined: Cryptococcus neoformans, comprising the grubii and neoformans varieties (genotypes VNI, VNII, VNIII, VNIV), and Cryptococcus gattii (genotypes VGI, VGII, VGIII, VGIV).10,17,26 Currently seven species are considered (even when that taxonomic basis is still controversial13) within the Cryptococcus neoformans–Cryptococcus gattii complex: Cryptococcus neoformans (C. neoformans var. grubii, VNI and VNII), C. deneoformans (C. neoformans var. neoformans VNIV), Cryptococcus gattii (C. gattii VGI), C. bacillisporus (C. gattii VGIII), C. deuterogattii (C. gattii VGII), C. etragattii (C. gattii VGIV), C. decagattii (C. gattii VGIV/VGII) and the intervariety hybrids C. neoformans×C. deneoformans hybrid (VNIII) and interspecies C. deneoformans×C. gattii hybrid (C. neoformans var neoformans×C.gattii VGI), C. neoformans×C. gattii hybrid (C. neoformans var grubii×C. gattii VGI) and C. neoformans×C. deuterogattii hybrid (C. neoformans var. grubii×C. gattii VGII).10 However, such taxonomic grounds are currently controversial, mainly due to the lack of clear biological differences between the lineages and of a clear consensus with respect to the limits and numbers of the putative species boundaries. As such, the various C. gattii lineages are collectively considered the C. gattii species complex.13
In Latin America cryptococcosis is frequent, although there is no data on the true incidence since reporting it is not mandatory in most countries of the region. However, the extrapulmonary involvement is strongly related to HIV infection, being the former a mandatory reportable disease. From the 223,100 cases of meningeal cryptococcosis that were estimated to occur globally in people living with HIV in 2014, Latin America occupied the third place in number of patients, with an estimated incidence of 5300 cases per year. Of these, Brazil and Colombia were the countries with the highest incidence, between 1001 and 2500 cases, followed by Argentina and Mexico with an incidence of 501–1000 cases.4,6,23 Meningeal cryptococcosis is the most frequent clinical presentation of this disseminated mycosis. Cutaneous manifestations are observed in approximately 5–7% of patients with systemic cryptococcosis and may have a variety of presentations, including papules, plaques and ulcers. On the other hand, lesions in the oral mucosa are extremely unusual.1
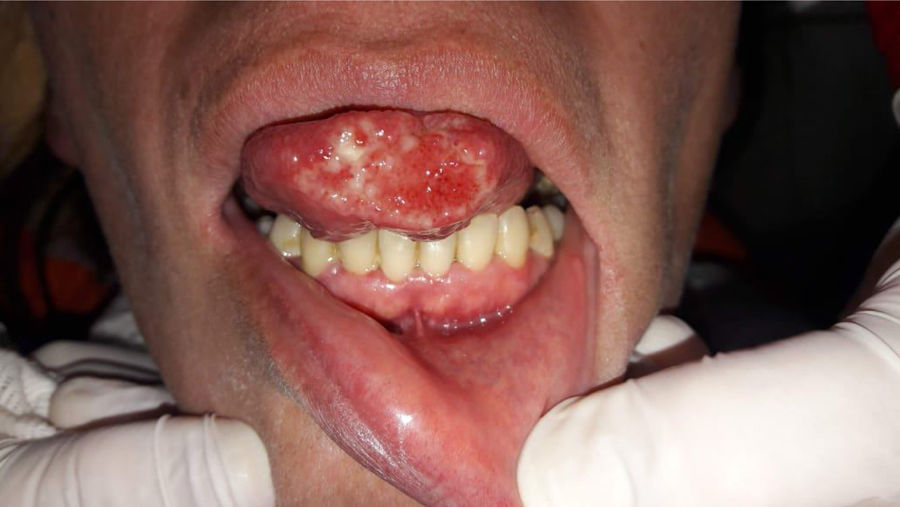
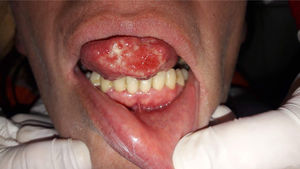
Case reportA 40-year-old female patient was assisted at the Mycology Unit of the F. J. Muñiz Hospital due to an ulcerative painful lesion on the tip of the tongue (Fig. 1). She had been referred from José Dueñas Odontology Hospital. The patient was born in Yuty (Paraguay), and lived for two years in Spain as a teenager; at that time she was residing in La Plata City, Buenos Aires, Argentina. Nine months prior to the medical consultation she suffered from a respiratory condition which resolved without any treatment and was considered an acute bronchitis. Although definite diagnosis was not established it could have been a cryptococcal primoinfection. Besides a mucosal ulcer on the tip of the tongue, her clinical exam did not reveal either palpable satellite adenopathies or other skin lesions. She related having lost weight, suffering cough with poor expectoration, and intermittent fever. At the time of the physical examination she was lucid, afebrile, without motor or meningeal focus. She was eupneic, normotensive, and the semiology of heart and lungs did not present any alteration. Abdomen was soft with no palpable visceromegaly. Chest radiography showed no parenchymal lesions. Chest tomography showed only isolated bronchiectasis in both lung bases, although the patient was not a smoker. Abdominal ultrasound did not show pathological findings. The results of laboratory analysis were the following: red blood cells count 3.75×106/μl; hemoglobin 10.7g/dl; hematocrit 32%; platelets 147,000/μl; white blood cells count 2200/μl; ESR 77mm 1st hour; glucose 83mg/dl; urea 25mg/dl; creatinine 0.66mg/dl; total proteins 7.8g/dl; albumin 3.9 g/dl; GOT 32 U/l; GPT 25 U/l; and LDH 323U/l. Chagas disease serology, HCV anti-IgG and VDRL were non-reactive; serology to treponemal antibodies was positive, as well as HBV anticore IgG. HBVs antigen test was non-reactive and HBVs anti-IgG concentration was 39IU/ml. Serology for HIV 1–2 (fourth generation ELISA) was reactive, and HIV 1–2 viral load was 589,000cop/ml 5.8 log10. TCD4+ lymphocytes count was 16cells/μl (4%), and TCD8+ 186cells/μl (42%).
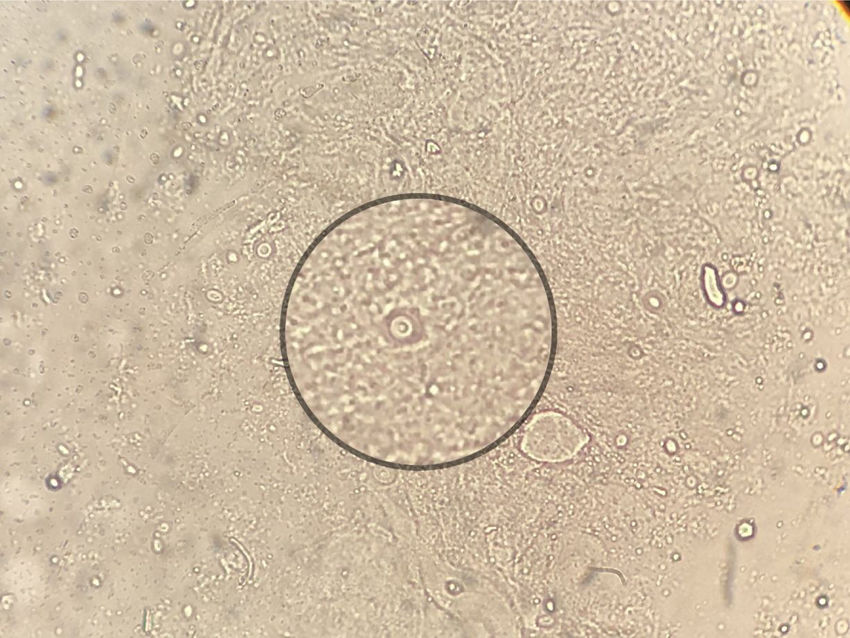
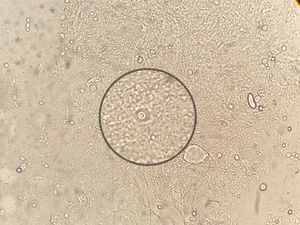
For the mycological exams a scarification from the tongue ulcer, as well as a biopsy, were taken.2 The wet mount microscopic examination of both samples showed encapsulated yeasts compatible with Cryptococcus (Fig. 2). The biopsy was cultured in Sabouraud agar, sunflower seeds agar, and brain-heart infusion (BHI) agar which were incubated at 28°C and 37°C for 15 days.9,20 Cultures were observed daily and colonies compatible with Cryptococcus were obtained. In the mycological study of sputum no fungal elements were evidenced in the microscopic examination, but a species of Cryptococcus was grown in the culture of this sample. The detection of cryptococcal capsular polysaccharide antigen in serum was performed by latex agglutination (LA) (CryptoLatex®, IMMY, Norman Kew Surrey, OK, USA) and by lateral flow assay (LFA) (IMMY, Norman Kew Surrey, OK, USA) techniques. Both yielded a positive result, but only with the undiluted serum.11 Due to the observation of encapsulated yeasts in the direct examination of the lingual mucosa, blood culture by lysis-centrifugation9,20 and brain CT scan without contrast were requested. The latter did not reveal pathological findings so lumbar puncture was performed to discard central nervous system involvement; the opening pressure registered was 12cm H2O. The physicochemical characteristics of the cerebrospinal fluid (CSF) were the following: proteins 0.51g/l, glucose 45mg/dl and no cells observed. Direct examination with Indian ink and CSF cryptococcal antigen test yielded negative results. After two weeks of incubation both blood and CSF cultures were negative.
The species identification was carried out by the ability of growing at 37°C, the presence of capsule, urease activity and the production of phenoloxidase. The differentiation between C. neoformans and C. gattii was performed by means of the ability of development in canavanine-glycine-bromothymol blue (CGB) and in Salkin (cycloheximide-glycine-phenol red) media.9,15,20 Genotyping was accomplished by means of a PCR-RFLP of the URA5 gene followed by double enzymatic digestion with Sau 96I and Hha I enzymes. Restriction fragments were separated in 3% agarose gel electrophoresis at 100V for 2h. The RFLP patterns were compared with the standards obtained from reference strains (C. neoformans var. grubii: CBS 10085 VNI and CBS 10084 VNII; C. neoformans hybrid AD: CBS 10080 VNIII; C. neoformans var. neoformans: CBS 10079 VNIV; and C. gattii: CBS 10078 VGI; CBS 10082 VGII; CBS 10081 VGIII and CBS 10101 VGIV). Our isolate was identified as C. neoformans var. grubii genotype VNI.17,26
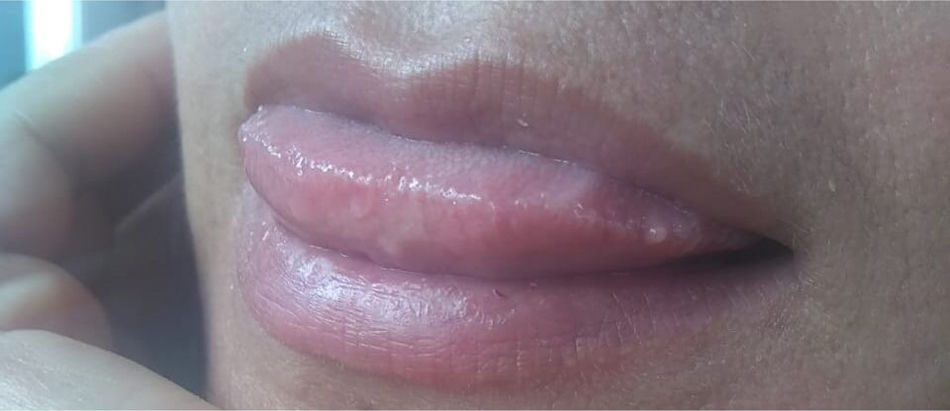
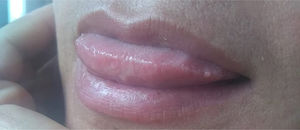
The patient was prescribed 800mg of oral fluconazole per day together with 160/800mg of trimethoprim/sulfamethoxazole during three weeks. After 4 weeks, she started the antiretroviral treatment with 800mg of darunavir and 100mg of ritonavir plus emtricitabine and tenofovir in daily doses of 300/200mg.19 After 8 weeks of antifungal treatment, the fluconazole dose was reduced to 400mg/day. Four weeks later, secondary prophylaxis (fluconazole 200mg/day) was set up with excellent clinical evolution (Fig. 3).
DiscussionAccording to the information provided by the National Reference Center INEI-ANLIS “Dr. Carlos G. Malbrán”, 1756 cases of cryptococcosis were diagnosed in Argentina between 2002 and 2012. Out of these, 573 (32.6%) were reported by the Mycology Network of Buenos Aires City. In 2010, 50% of the cases from Buenos Aires were diagnosed at the F.J. Muñiz Infectious Diseases Hospital, representing 15% of the cases registered in the country. The National Network of Mycology Laboratories of Argentina conducted five retrospective surveys on fungal infections in 2002, 2004, 2008, 2010 and 2012, where cryptococcosis ranked second in prevalence when considering deep mycoses.16 The first case of AIDS related to cryptococcosis in the formerly mentioned Muñiz Hospital was diagnosed in 1983. In the 1990s about 3 new cases were diagnosed weekly and in the last 5 years an average of 60 new patients have been diagnosed per year. Currently, it is still the most frequent systemic mycosis in HIV-infected patients, followed by pneumocystosis and histoplasmosis.1
Among the most frequent differential diagnoses of tongue ulcers, sores, syphilitic chancre, neoplasms, histoplasmosis and paracoccidioidomycosis are included.2 Cryptococcosis causes skin lesions in a low percentage of cases, mostly appearing as molluscoid or ulcerated papules more often located on the neck or face. The mucous membrane manifestations are really exceptional. The first case of cryptococcosis in an HIV positive patient with mucosal involvement was published in 1987 in Houston, Texas.14 This severely immunosuppressed patient suffered from an ulcer on the lateral side of the tongue produced by Cryptococcus, and concomitantly suffered from a disseminated atypical mycobacteriosis and mucosal Kaposi sarcoma. From that first case to date, publications of this type of clinical presentation are very infrequent.12 Cases related to other types of immunodeficiency have also been observed.22 (Table 1) In Argentina, only one case of an HIV positive patient with an ulcerated lesion on the hard palate has been published.8 It is important to bear in mind that extrapulmonary cryptococcosis lesions are AIDS defining or should be linked to a deficit of cellular immunity. In contrast, it should be taken into account that when C. gattii is the species involved, the immunodeficiency may not be found since this species is more aggressive or virulent and may cause these lesions in immunocompetent persons.21C. neoformans is, approximately, eight times more frequently isolated than C. gattii (88.6% versus 11.4%). The proportion C. neoformans/C. gattii is variable among continents, with 68:1 in Europe, 33:1 in Africa, 7.6:1 in Asia, 4.5:1 in Central and South America, 3.5:1 in North America and 1:1.5 in Oceania, where C. gattii is the predominant species.3 In the present case, the etiologic agent was C. neoformans genotype VNI, like most of the isolates from HIV positive patients in Argentina.17,24,26
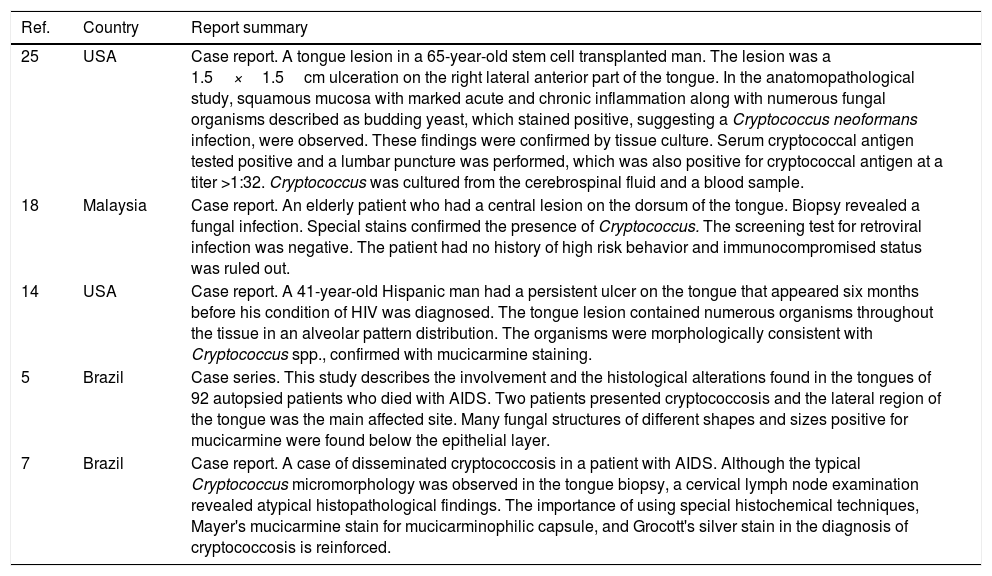
Results of the literature review on tongue lesions by Cryptococcus spp.
| Ref. | Country | Report summary |
|---|---|---|
| 25 | USA | Case report. A tongue lesion in a 65-year-old stem cell transplanted man. The lesion was a 1.5×1.5cm ulceration on the right lateral anterior part of the tongue. In the anatomopathological study, squamous mucosa with marked acute and chronic inflammation along with numerous fungal organisms described as budding yeast, which stained positive, suggesting a Cryptococcus neoformans infection, were observed. These findings were confirmed by tissue culture. Serum cryptococcal antigen tested positive and a lumbar puncture was performed, which was also positive for cryptococcal antigen at a titer >1:32. Cryptococcus was cultured from the cerebrospinal fluid and a blood sample. |
| 18 | Malaysia | Case report. An elderly patient who had a central lesion on the dorsum of the tongue. Biopsy revealed a fungal infection. Special stains confirmed the presence of Cryptococcus. The screening test for retroviral infection was negative. The patient had no history of high risk behavior and immunocompromised status was ruled out. |
| 14 | USA | Case report. A 41-year-old Hispanic man had a persistent ulcer on the tongue that appeared six months before his condition of HIV was diagnosed. The tongue lesion contained numerous organisms throughout the tissue in an alveolar pattern distribution. The organisms were morphologically consistent with Cryptococcus spp., confirmed with mucicarmine staining. |
| 5 | Brazil | Case series. This study describes the involvement and the histological alterations found in the tongues of 92 autopsied patients who died with AIDS. Two patients presented cryptococcosis and the lateral region of the tongue was the main affected site. Many fungal structures of different shapes and sizes positive for mucicarmine were found below the epithelial layer. |
| 7 | Brazil | Case report. A case of disseminated cryptococcosis in a patient with AIDS. Although the typical Cryptococcus micromorphology was observed in the tongue biopsy, a cervical lymph node examination revealed atypical histopathological findings. The importance of using special histochemical techniques, Mayer's mucicarmine stain for mucicarminophilic capsule, and Grocott's silver stain in the diagnosis of cryptococcosis is reinforced. |
Regarding treatment, the guidelines and recommendations for meningeal cryptococcosis are very well established but in disseminated forms without central nervous system compromise evidences are inconclusive; starting an antiretroviral treatment is neither clear. In meningeal cryptococcosis, antiretroviral treatment should be started at least four weeks after the beginning of the antifungal therapy, thus reducing the possibility of complications (as immunological reconstitution syndrome) due to ART starting out. The possibility of an inflammatory response related to ART in a fungal infection, viable or not, might cause an increase in intracranial pressure of such magnitude that could result in the patient's obit. In non-meningeal forms it is unnecessary to delay the ART, but the CFS culture must be checked throughout two weeks at least.23,27 In meningeal cryptococcosis, amphotericin B plus 5-fluorocytosine or fluconazole are used for induction. In mild or moderate cases of non-meningeal forms, fluconazole is used in hemodynamic and clinically stable patients. Both amphotericin B and fluconazole are truly effective drugs for cryptococcosis; a combined treatment in meningitis is preferred since it is a high-mortality condition, while in non-meningeal forms the use of amphotericin B is not usually prescribed in order to avoid its side effects.23,27 As it has been described, the patient received P. jirovecii prophylaxis because pneumocystosis has an incidence greater than 10% in our country, so primary prophylaxis is recommended. In patients with CD4+ T lymphocytes<200cells/μl, trimethoprim+sulfamethoxazole 160/800mg orally administered, three times a week, is recommended.
The few cases published worldwide about mucosal involvement caused by Cryptococcus in HIV positive patients were observed in patients with CD4+ T lymphocytes count less than 100cells/μl. The early diagnosis of this disease is very important for a favorable evolution. Currently, the WHO recommends the detection of the cryptococcal capsular polysaccharide antigen in serum, by LFA technique, prior to starting antiretroviral treatment in patients with a CD4+ T cell count less than 100cells/μl to avoid immunological reconstitution inflammatory syndrome.27 Unfortunately, we still have patients who know their immune status for the first time in the context of an opportunistic disease.
We conclude that the cytodiagnosis study of the cutaneous or mucosal samples remains a rapid, low-cost and very effective test. Although the involvement of the oral mucosa is uncommon in this disease, it is important to include it in the differential diagnosis of mucosal lesions in HIV positive patients.