This review summaries the available research on nuclear magnetic resonance to show the Auriculotherapy (AA) effect in human brains. Through search and analysis of clinical research in the PubMed/MEDLINE database, available related articles up to January 2023 were used only in English. This overview is not comprehensive and is due to be predominantly informative. A total of 4 trials met our inclusion criteria and were included in the quality analysis. Electrical stimulations were more frequent (3 studies) than needles (1 study). The above research delivers insufficient evidence that the AA at different points led to other brain structures' modulation. Although, the brain structures activated by the AA seems to overlap territories belonging to the anxiety's neurobiology. The endeavor to link the AA mechanisms to the cranial nerves combined with an embryological approach is promising. Nevertheless, additional studies with imaging, different AA techniques, and auricular points are necessary to provide evidence for this effect on mental health.
Esta revisión resume la investigación disponible sobre resonancia magnética nuclear para mostrar el efecto de la Auriculoterapia (AA) en cerebros humanos. A través de la investigación y el análisis de la investigación clínica en la base de datos de PubMed/MEDLINE, se utilizaron los artículos relacionados disponibles hasta enero de 2023 solo en inglés. Esta perspectiva no es amplia, y parece ser predominantemente informativa. Un total de 4 ensayos cumplieron nuestros criterios de inclusión, y fueron incluidos en el análisis de calidad. Las estimulaciones eléctricas fueron más frecuentes (3 estudios) que las de las agujas (1 estudio). La investigación anterior aporta evidencia insuficiente en cuanto a que la AA en diferentes puntos originó una modulación de otras estructuras cerebrales, a pesar de que las estructuras cerebrales activadas por la AA parecen solapar territorios que pertenecen a la neurobiología de la ansiedad. El esfuerzo por vincular los mecanismos de la AA con los nervios craneales junto con un enfoque embriológico es prometedor. Sin embargo, son necesarios estudios adicionales con técnicas de imagen, diferentes técnicas de AA, y puntos auriculares para aportar evidencia para este efecto en la salud mental.
According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), anxiety is the future anticipation of unbalanced, often associated with muscle tension and caution in preparation for future hazards.1 The excess may trigger severe psychopathological events disturbing an individual's everyday life.2 Anxiety disorders are highly prevalent, with global estimations varying from 3.8 to 25% across countries, with incidence rates as high as 70% in people with chronic health conditions.3
Succeeding the National Institute for Health and Care Excellence regarding pharmacological therapies for anxiety disorders, such as Benzodiazepines and antipsychotics, are associated with tolerance, dependence, and linked with several adverse effects.4 Therefore, they should not be used regularly to manage anxiety disorders.4 Conventional medicine, such as psychotherapy and medication, has successfully treated anxiety disorders; however, those patients with anxiety struggling to achieve complete remission of their condition feel the need to use Complementary and Alternative Medicine (CAM).5
The National Center for Complementary and Integrative Health classified CAM as respects: i) Nutritional (e.g., special diets, dietary supplements, herbs, probiotics, and microbial-based therapies), ii) Psychological (e.g., meditation, hypnosis, music therapies, relaxation therapies), iii) Physical (e.g., acupuncture, auriculotherapy, massage, spinal manipulation) and iv) combinations such as psychological and physical (e.g., yoga, tai chi, dance therapies, some forms of art therapy) or psychological and nutritional (e.g., mindful eating).6
Auriculotherapy (AA) is a therapy somewhat based on the ancient Chinese practice of body acupuncture, originating from a French physician's discoveries in the 1950s (Dr Paul Nogier and colleagues) demonstrating that specific areas of the external ear were associated with pathology in particular body parts.5,6 Typically, AA involves the insertion of needles6 (e.g., semipermanent or metal filiform needles and bloodletting) or pressure7 (e.g., using seeds, massage, or magnetic stones) or heating (e.g., moxibustion)8 or by stimulation as laser therapy,6 and electrostimulation9 in the auricular skin for therapeutic purposes.10,11
It is a technique known to diagnose and treat physical and psychosomatic dysfunctions by stimulating a specific point in the ear.12 In 2018, only fourteen systematic reviews with high methodological quality were published about AA, which focused on managing insomnia, smoking cessation, and pain.13 Since then, exponential research has grown, describing it as a strategy capable of adjusting signal processing in the central nervous system,14 and exploiting brain plasticity,15 which is the perfect complementary technique to address psychological impairments. Curiously, AA evaluated with anxiety scales had a positive effect on pre-operative anxiety.16–22 The salivary cortisol was reduced in students before an exam compared with the control group,22–24 and in post-cesarean woman.25 Regarding generalized anxiety disorder, Rivadeneira et al. (2015) reported AA was more effective than conventional drug in decreasing the Self-Assessment Inventory and anxiety symptoms remission after four weeks, which could potentially reduce the use of psychopharmacologic drugs.26
This narrative review aims to clarify the relationship between the possible auriculotherapy's neuromodulators' effects on anxiety management. As the current understanding of the mechanisms behind AA stands on the embryological hypothesis and the solid innervation of the ear, its relevance stands out in summarizing existing research and supporting future research using AA.
MethodsThe authors first identified studies through keywords search “ear or auricular” and “fMRI” using Pubmed/Medline. Articles were collected from inception to January 2023, in English, and the outcome was the fMRI bold signal activation and signal reduction in humans. We excluded all guidelines for treatments, surveys, case series, case reports, interrupted time-series trials, qualitative trials, trials with missing or incomplete data, cohort studies, reviews, conference abstracts/posters, expert opinion, ‘duplicate publications’, newspaper articles, book reviews, ‘mass media publications’, health publications, general comments, or letters, due to their potential high risk of bias.27 This overview is not comprehensive and is due to be predominantly informative.
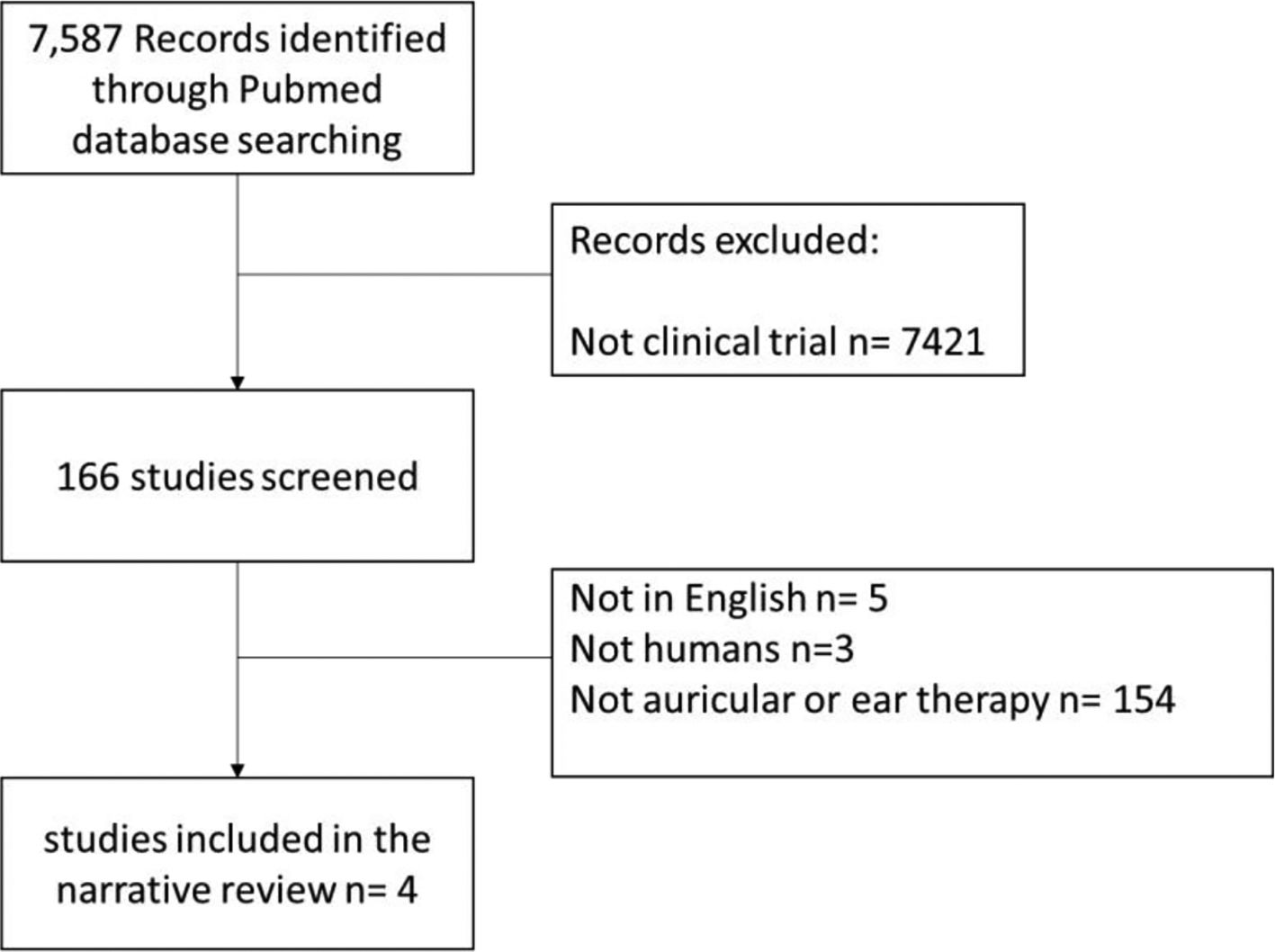
ResultsOur search identified 7587 citations where only 166 titles were reviewed, as shown in the diagram flow (Fig. 1). A total of 4 trials met our inclusion criteria and were included in the quality analysis.
The 4 trials included two different type of AA interventions retrieved, where electrical stimulations were more frequent28–30 than needles.31
In two different sessions, the author's Zhang et al. (2019) applied auricular electrical stimulation to twenty-six patients with migraines. The authors reported that the left cymba concha area produced significant fMRI signal reductions at the LC and improved signal in other brain areas (e.g., right temporoparietal junction, right para-hippocampus, left secondary somatosensory cortex, and left amygdala) compared to the left tail of the helix area.28
Badran et al. (2018) enrolled 17 healthy adults who received either left tragus or earlobe electric stimulation. They documented the tragus area activates the cerebral afferents of the vagal pathway (e.g., the Contralateral postcentral gyrus, bilateral insula, frontal cortex, right operculum, and left cerebellum), while the earlobe stimulation only produced activation in the contralateral postcentral gyrus.29
Kraus et al. (2013) investigated fMRI effects in response to electrical stimulation of two zones in the left outer auditory canal.30 Eight healthy subjects received electrical stimulation at the anterior wall area, and the other eight participants received it at the posterior side of their left external auditory canal, and both groups were later stimulated on their corresponding ear lobe.30 The authors found the anterior part of the auricular stimulation decreased fMRI bold signal in the locus coeruleus and the solitary tract compared with the ear lobe region.30 In contrast, the stimulation at the posterior wall leads to unspecific changes in the bold signal within the solitary tract.30
M Romoli et al. (2014) deliver preliminary evidence on the specificity of two auricular acupoints, the “Brain Stem Auricular Acupoint” and the “Thumb Auricular Acupoint” in 6 healthy volunteers who experienced two fMRI sessions.14 These authors reported that the needle in the “Brain Stem Auricular Acupoint” of the left ear showed a pattern that largely overlapped regions belonging to the pain matrix.14 At the same time, the “Thumb Auricular Acupoint” increased activation bilaterally in the parietal operculum, the territory of the secondary somatosensory area.14
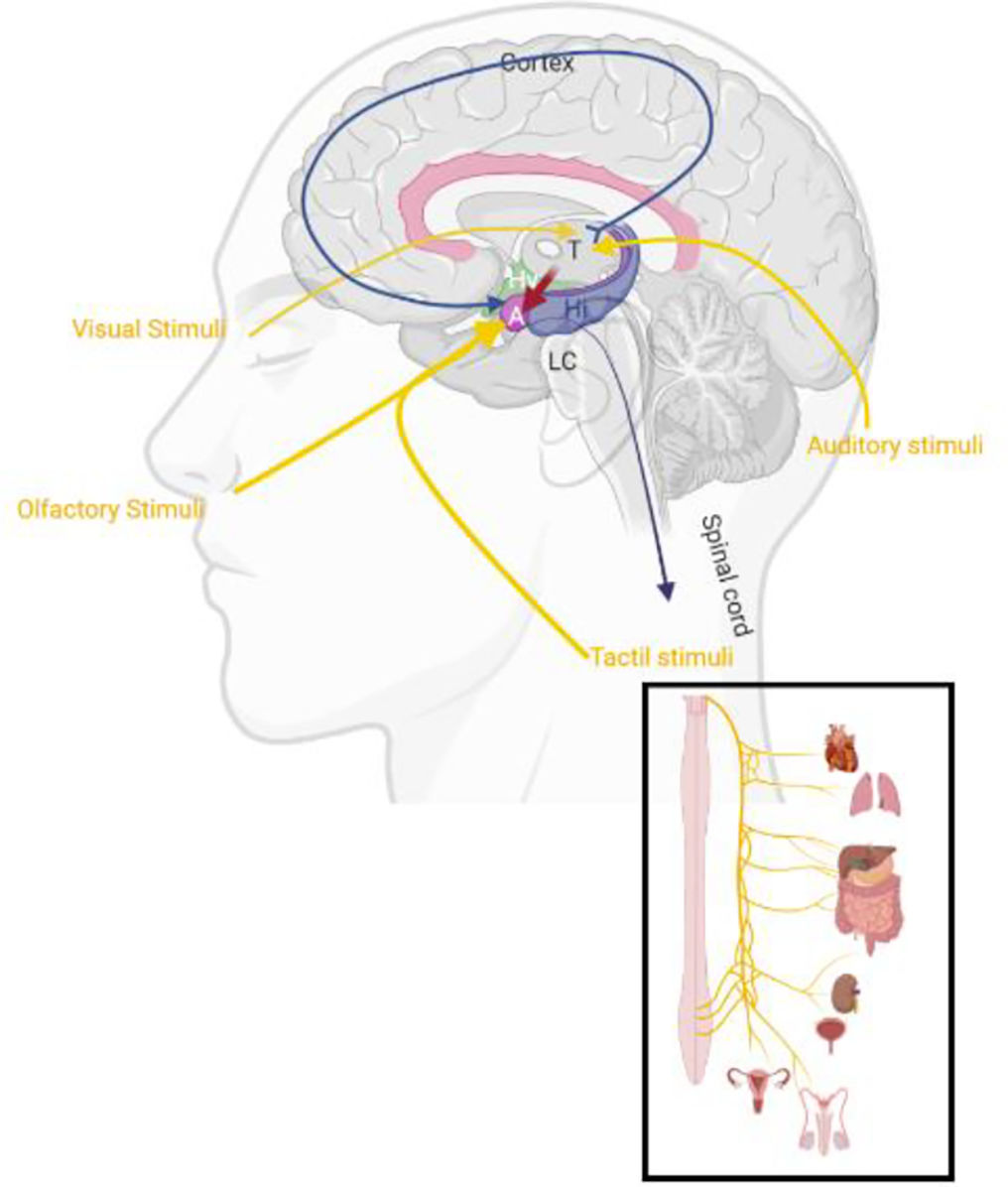
DiscussionMany neurotransmitters have been connected to anxiety´ neurobiology, including serotonin,32 Gamma-aminobutyric acid (GABA),33 Norepinephrine (NE),34 and dopamine.35 Curiously, disbalances of any hormone may also affect the balance of activity in the brain's emotional centers in distinct ways.36 However, cause and effect are rarely known, and it's often unattainable to distinguish between inadequate neurotransmitter balance (e.g because of life experiences) or underlying genetic predisposition.37,38 Both can happen to anyone with anxiety; in some cases, a mix of both may be responsible for it. The limbic system processes sensory input from the external and internal environment (Fig. 2) to determine, through memory and motivation, the emotional, autonomic, motor and cognitive responses essential for self-preservation and survival.39 The emotional circuit can be influenced by many chemical messengers implicated in anxiety psychopathology (such the co-release of neurotransmitters and neuropeptides) which are represented in limbic regions in the central nervous system (CNS).38 One of those chemical messengers contain cholecystokinin, a peptide hormone found in the gastrointestinal system responsible for stimulating the digestion also responsible to inhibit the vagus nerve (VN).40
Schematic illustration from the sagittal view of slice representing a normal limbic system midline connection after visual, olfactory, tactile, and auditory stimulus. Legend: LC: Locus coeruleus, A: Amygdala, Hy: Hypothalamus, T: Thalamus, Hi: Hippocampus. Schematic illustration created with BioRender.com.
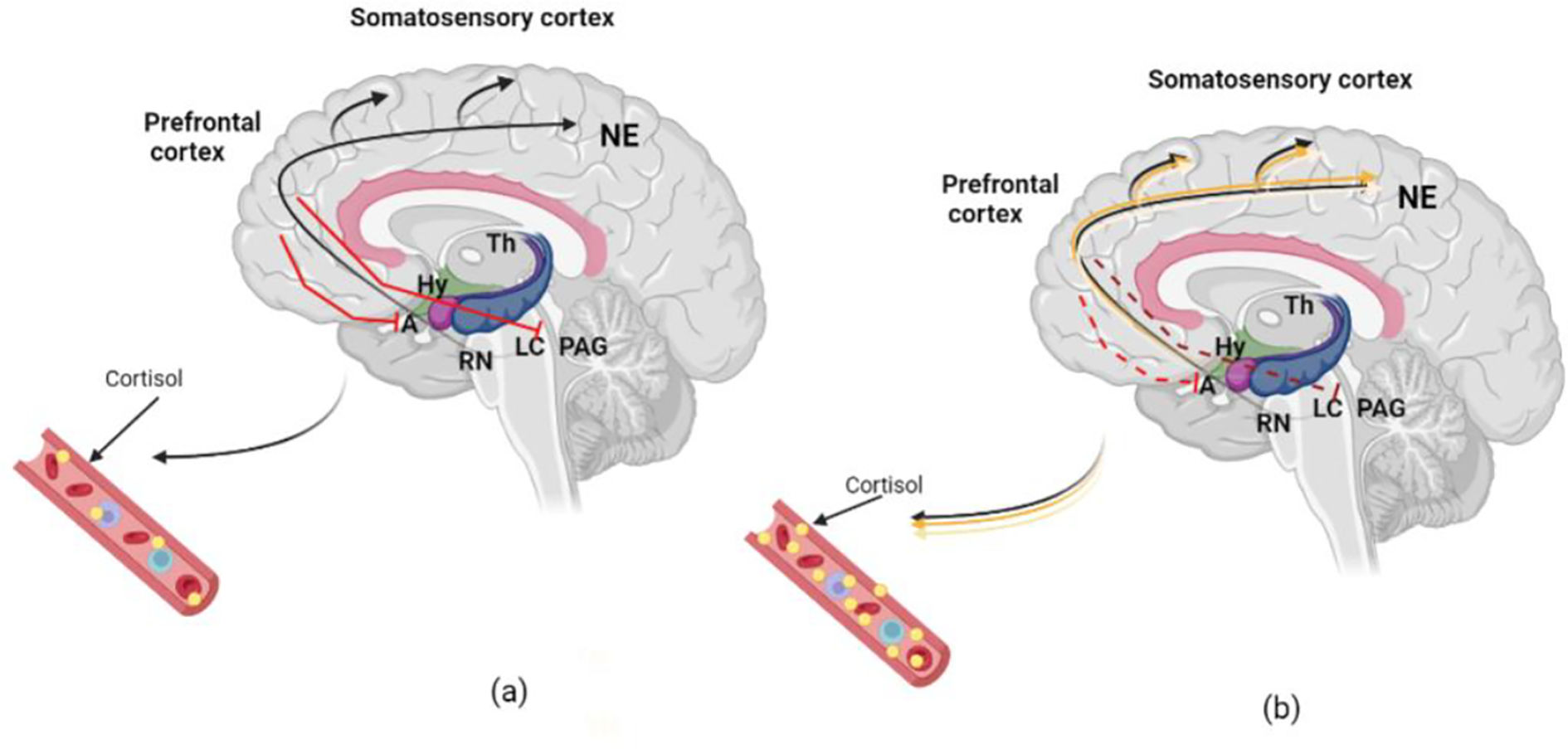
By receiving projections directly from olfactory and tactile stimulus and indirectly (through thalamus or somatosensory cortex) by auditory and visual areas, the amygdala (Fig. 3) mediates threats and other emotional responses including the regulation of the cortisol via hypothalamus.41 Potential threats identified by the sensory system (e.g., anxiety) appear to also trigger the locus coeruleus (LC),42 the main supplier of NE in the CNS.43 The secretion of NE from the LC reaches the amygdala and the prefrontal cortex. This pathway affects HPA activation by producing cortisol, which elevates glucocorticoid distribution for a coordinated physiological reaction44 (Fig. 3a). While the cortisol released from the adrenal cortex allows the body to persist on high alert,45 the disproportionate concentration of these structures is associated with a prolonged anxious state.46 Combined with this amplified response, there can also be deficient HPA regulation, leading to an inability to control the physiological response (Fig. 3b). Further functional magnetic resonance imaging (fMRI) will hopefully allow human LC assessment and a better understanding of its role in anxiety‘s neurobiology.47
a) Standard organism modulation response to acute physiological input. Legend: The red lines reveal the global and the Locus coeruleus (LC) regulation by the secretion of noradrenalin (NE) reaching the amygdala (Amy) and the Prefrontal cortex. This path affects Hypothalamic–pituitary–adrenal (HPA) activation by producing cortisol. (b) Abnormal organism reaction facing chronic physiologic input. Chronic anxiety can lead to less HPA regulation (dotted red lines). Therefore, increased NE in LC, amygdala, and hippocampus will deliver excessive cortisol production and contribute to reduced regulation of pathological anxiety. Fig. created with BioRender.com based on Morris, McCall, Charney, & Murrough (2020) work.48 (For interpretation of the references to color in this fig. legend, the reader is referred to the web version of this article.)
AA has been applied more systematically in Europe since Doctor Nogier presented the inverted fetus map conception in 1957.6 Nogier also found that the stimulation of the auricle leads to fluctuations in the radial artery (e.g., heart rate) known as “Vascular Autonomic Signal”.10,49 Paul Nogier and later Frank Bahr explained that the ear has a so-called somatotopy, meaning that the whole person is represented on the ear.10,49 Based on his AA experience, Paul Nogier began to take his patients' pulses while examining their ears. He discovered variations of the pulse when he touched different ear zones. This variation of pulse, or vascular autonomic signal, is a reaction from the CNS (sympathetic and parasympathetic) after auricular points stimulation.50 The ear reflex map of a French physician, Paul Nogier, was introduced to China in 1958 by Dr. Ye Xiao-Lin.31 Since then, numerous reports arrived founding AA helpful for tooth extraction and sciatica management conducted by ear cauterization.
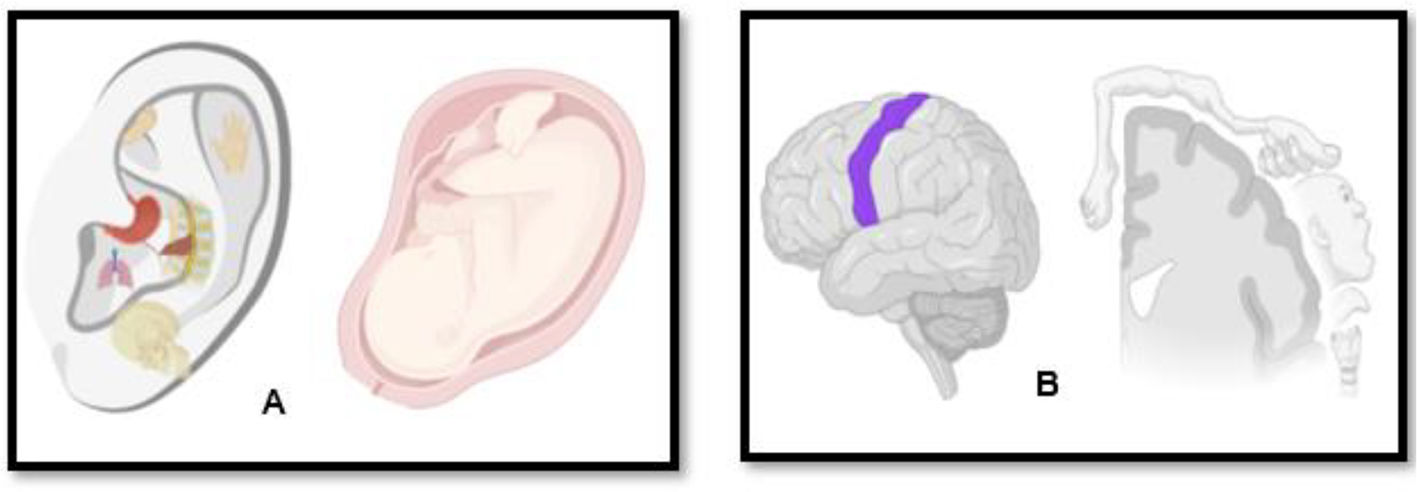
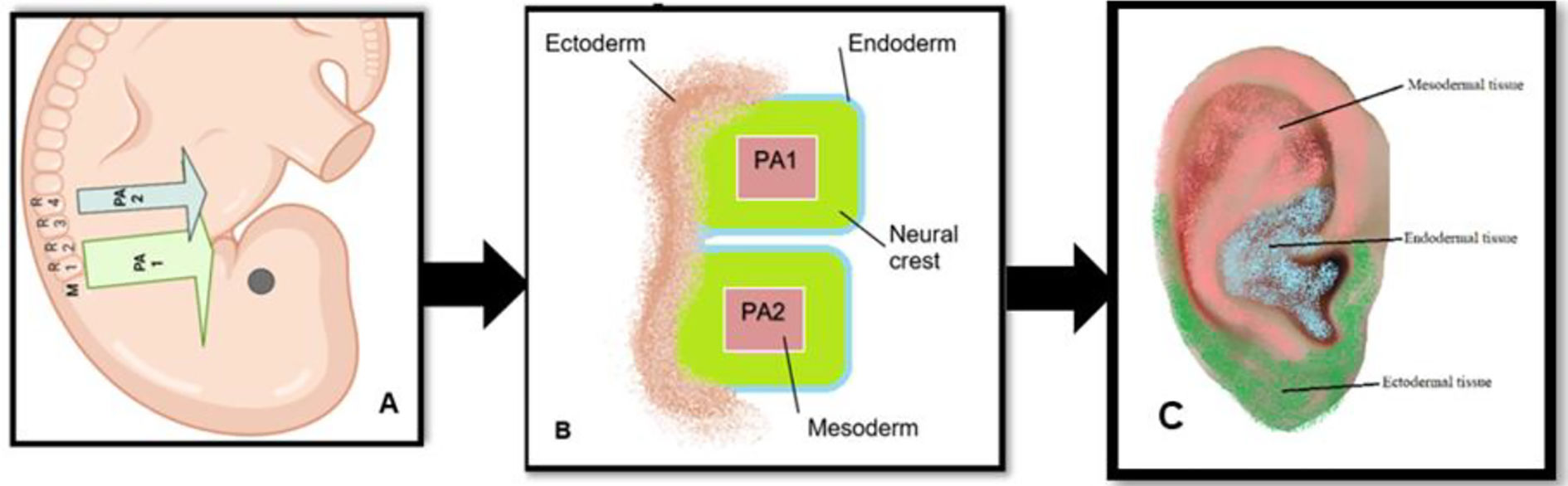
AA books sometimes refer to an embryological hypothesis which strives to explain how the ear's reflex map relates to the rest of the body.12,51–53 On the one hand, the embryological view is based on the somatotopic organization of the human fetus theory6 in the auricle (Fig. 4A) and the adult ear's nerve network (Fig. 4B). On the other hand, it uses a general anatomical understanding of how organs and tissues grow from the fetus's three germinative layers.
A. Schematic representation of a fetus in the intrauterine position projected on the pinna exemplifying Paul Nogier and Frank Bahr somatotopic theory, where the whole person (and its organs) is represented on the ear; B: Schematic representation of the regions of the human body reflected in the cerebral cortex - somatotopic map of the brain, simulating the Penfield homunculi theory. Combining both theories, when auriculotherapy is performed is believed the specific point chosen by the acupuncturist is correlated with the somatotopic cortex in the brain. Fig. created with BioRender.com.
The ears are not an exemption; it originates from the proliferation of organized embryonic tissue of undifferentiated cells.54 In all vertebrates, the pharyngeal arches developed from the cephalic portion of the neural crest (e.g., strip of tissue that runs down the back of the origin and gives genesis to a large number of different organs) are responsible for producing the cartilage, bone, nerves, muscles, glands, and connective tissue of the face and neck.55 The pharyngeal arches are banded externally by ectoderm and internally by endoderm and are filled with neural crest cells (Fig. 5) surrounding a mesodermal core.53,55,56
Schematic illustration the early set-up of the ear. Legend: (A) The pharyngeal arches (PA) are filled by neural crest streams; the first PA1 is filled with neural crest cells from the midbrain (M), rhombomere (r1) and r2, while the second PA2 is filled with crest predominantly from r4. (B) The first and second PA are divided internally by the endodermal and externally by the ectodermal tissue. (C) division of the embryological cells in auricular area theory created based on Anthwal., N and Thompson.,H (2015); Liu C et al., (2020) and Terry Oleson (2014) works. Schematic illustration created with BioRender.com.
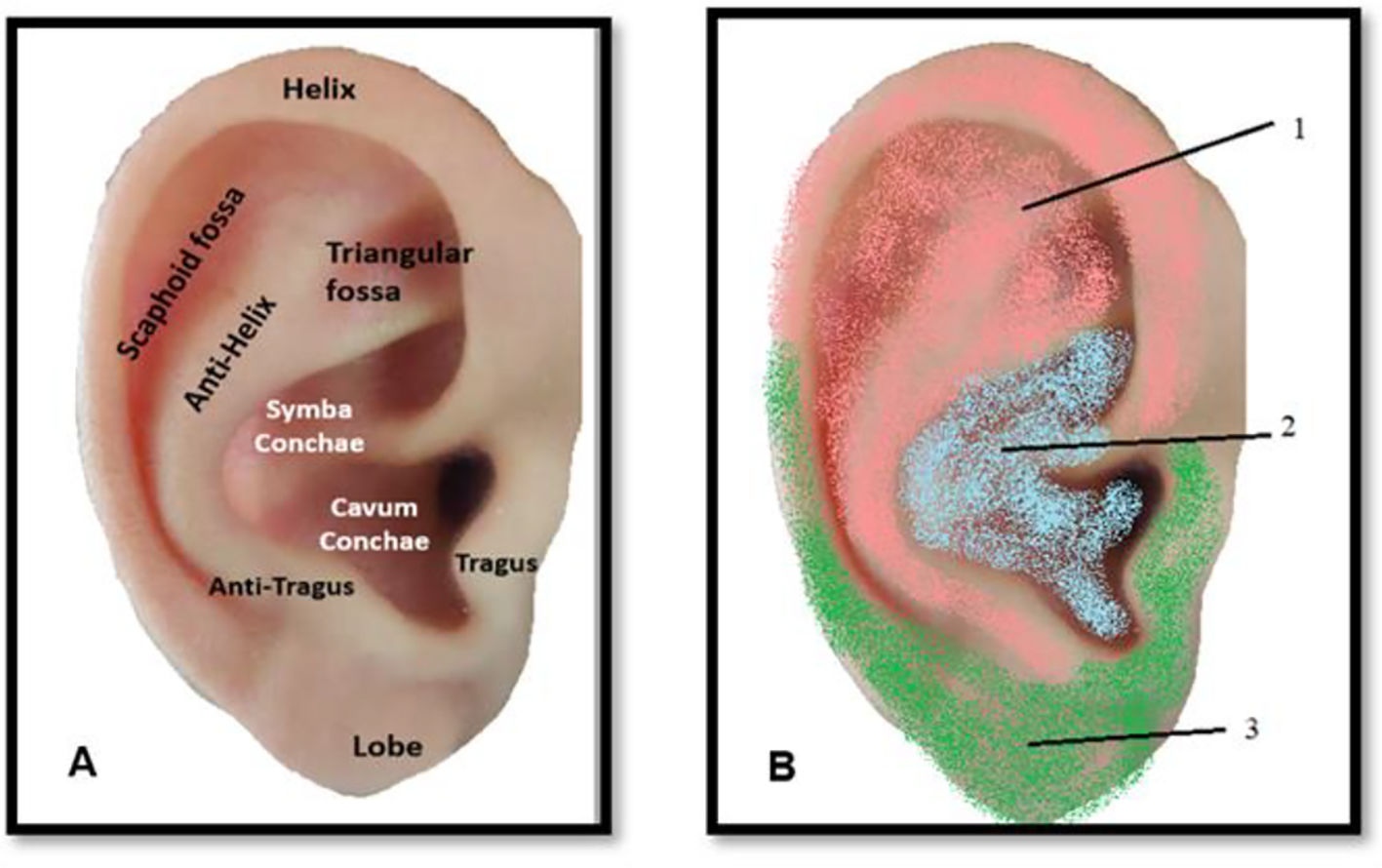
These germinative layers become specialized into different types of organs. For example, the endoderm develops most internal organs: the stomach and intestinal system, lungs, tonsils, liver, pancreas, bladder, urinary system, thyroid, parathyroid and thymus represented in the concha (Fig. 6A); innervated by the vagus nerve. The ectoderm creates the skin, the brain, the spinal cord, the subcortex, cortex and peripheral nerves, the pineal gland, the pituitary gland, kidney marrow, hair, nails, sweat glands, cornea, teeth, the mucous membrane of the nose, and the lenses of the eye, innervated by a branch of plexus cervicalis (Fig. 6B), which has indirect connections with the cortex.53,56 The mesoderm origins the skeletal muscles, smooth muscle, blood vessels, bone, cartilage, joints, connective tissue, endocrine glands, kidney cortex, heart muscle, urogenital organ, uterus, fallopian tube, testicles and blood cells from the spinal cord and lymphatic tissue, represented by the trigeminal nerve (TN).57 This reflects the scientifically validated organization and inverted orientation of the homunculus in the cerebral cortex as developed by Penfield.58 However, the body reflected in the brain does not illustrate the exact proportions of the actual body, such as Penfield's Homunculus. In 1937, Penfield and Boldrey described their work on the stimulation effects of the cerebral cortex in man. The homunculus can be considered as some form of “map” of human cortical representation, being more or less precisely in relation to actual brain areas identified at surgery.59 The same organization is thought to happen in the auricle, the acupuncture points corresponding to reflexes with body organs would be divided according to an organized anatomical arrangement. Thus, the head and hand occupy a larger area than they would if they were proportionate, while the thigh bone and arm reach a small space in the ear, precisely as in the somatotopic map of the brain. Consequently, the brain's somatotopic map is related to its functional importance rather than its actual physical size.
Schematic illustration about the human auricle anatomy. Legend: (A) and auricular innervation (B), 1: pink area exemplifies the trigeminal, and auriculotemporal nerve innervation; 2: includes the auricular branch of the vagus nerve (blue shading); 3: Green area is represented by the greater auricular nerve, desenvolved from Liu C et al., (2020) and Terry Oleson (2014) works. (For interpretation of the references to color in this fig. legend, the reader is referred to the web version of this article.)
Between the epidermis, dermis, and hypodermis are around 10.000 unique sensory receptors (including exteroceptors, interceptors and proprioceptors), at most 0.1 mm large.60 These sensory receptors are mainly made of collagen fibers wrapped by a plasma membrane containing a negative electrical charge (low acid dissociation values of the lipid head group).61 Each auricular point includes a group of these receptors62 served by the same nerve and blood vessel branch (majority from the external carotid artery),63 which are activated by mechanical pressure (e.g., seeds, massage, needle), heat (e.g., moxibustion) and stimuli such as laser light and electrical stimulation.64 According to Gate Control Theory by Melzack and Wall,65 the receptors deliver the information to the brain based on the stimulation location, pressure-generation which triggers the small myelin nerves to conduct stimulations to spinal cord, midbrain, hypothalamus and pituitary axis where therefore, neuropeptides are released.66,67
The information provided by thermal, algic and proprioceptive stimuli is transmitted from the auricular pavilion by the fibers by a great deal of overlap between multiple nerves: i) auriculotemporal nerve; ii) auricular branch of the VN; iii) greater auricular nerve (GAN) and minor occipital nerve (sensitive branch of the cervical plexus).12 The auriculotemporal nerve originates from the mandibular branch of the TN, which mainly supplies the antero-superior and antero-medial areas of the external ear. Auricular branch of the VN is the only peripheral branch of the vagus nerve, covering a considerable part of the auricular area and most of the region around the auditory canal. The minor and greater occipital nerve originates from the C-2 branch of the cervical plexus, being responsible for the sensitive innervation of the upper third of the auricle. Finally, the major auricular nerve originates from the C-2 to C-3 branches of the cervical plexus, being responsible for the innervation of the lower region of the auricle.68
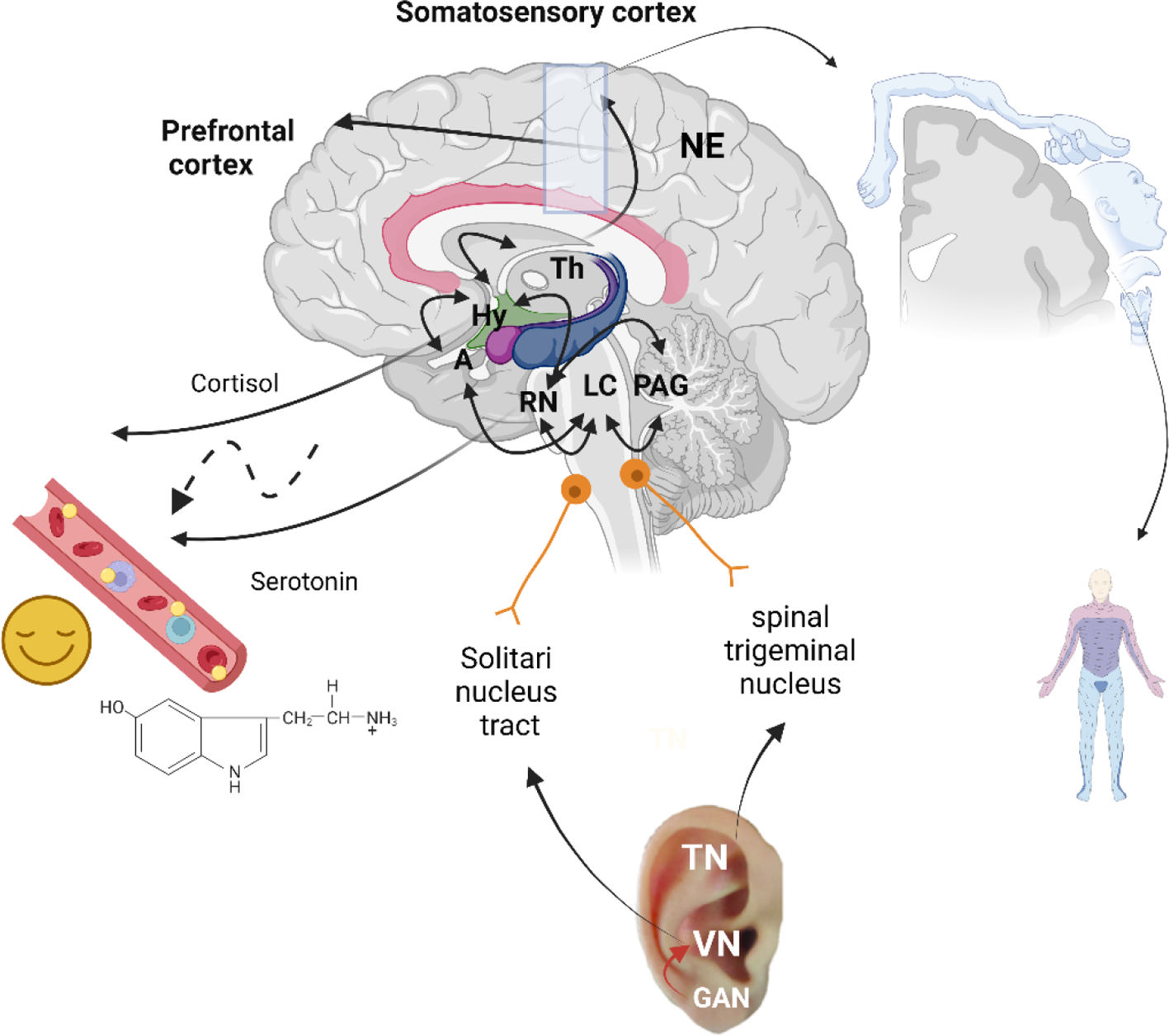
The VN is a mixed nerve composed of 20% “efferent” fibers (sending signals from the brain to the body) and 80% “afferent” (sensory) structures (carrying information from the body to the brain).69 The efferent cholinergic fibers are the main parasympathetic component of the autonomic nervous system, but a vital function of the VN is transmitting and mediating sensory information from throughout the body to the brain.70 The VN is the most extensive cranial nerve from the brain stem to the abdomen,71 with a somatic afferent distribution to the external auricular area. The VN contains direct and indirect connections to the cortical–limbic-thalamic-corpus callosum neural circuit relevant to emotional and cognitive functions in psychological disorders.72,73 The association between VN stimulation effects and AA was highlighted by procedures developed to electrically stimulate the VN through the AA points found in the auricle's concha region to treat depression74,75 and epilepsy.76,77 The VN stimulation seems to boost the NE release in structures responsible for regulating autonomic function and improve levels of neurotransmitters (e.g., GABA) in the brain and spinal cord while reducing glutamate levels.78
On the other side, TN stimulation has also been suggested as an explanation via AA, providing anatomical support for physiologic results where the TN stimulation affects brainstem structures,68 and in turn, influence forebrain areas involved in the pathophysiology of specific CNS disorders.
TN is responsible for perceiving touch and pain between the anterior ear site to the CNS via the spinal trigeminal nucleus (SpV).57 Interestingly, unlike the VN, the TN does not contain autonomic fibers.71 However, the impulses from the Spv reach extra-trigeminal regions in the CNS, including the posterior parietal, auricular, occipital areas, and thalamus, essential for controlling many autonomic functions.52 Besides that, SpV transmits nociceptive and tactile stimulus to the thalamus reaching the somatosensory cortex. It also has projections for the nucleus tractus solitarius (NST), the LC and the Raphe nucleus (RN). In the brainstem, the vagus afferent fibers end in the NST which has fibers linking directly or indirectly different brain regions [83]. These regions include the dorsal raphe nuclei, locus coeruleus, amygdala, hypothalamus, thalamus, and orbitofrontal cortex. The NST, located in the dorsomedial medulla, is the first brain structure responsible for conducting visceral and taste afferents carried by the cranial nerves and has a critical role in the initiation and integration of a wide variety of reflexes controlling cardiovascular function, respiration, and gastrointestinal motility [84,85]. The RN which contain primarily serotonergic neurons, release serotonin, as well as synaptic connections with the limbic system [87]. Moreover, the RN receives catecholaminergic innervation from the periaqueductal gray (PAG), amygdala, and hypothalamic nuclei, supporting those systems with mood regulation [88].
During the last years, some fMRI studies revealed different central nervous activations in distinct auricular areas accessed via electrical stimulation28–30 and by titanium semi-permanent needles of the external ear.
Electrically, the stimulation on the inner side of the tragus showed by fMRI decreased activation in limbic brain areas (including the amygdala, hippocampus, and para-hippocampal gyrus) and the middle/superior temporal gyrus but increased activation in the insula, precentral gyrus and the thalamus.29,30,73 Also, electrical stimulation at the cymba concha produced deactivations bilaterally in the hippocampus and hypothalamus,28 and activation of the conventional vagal projections. While the stimulation led to unspecific activation patterns; precisely in the ventral region of the caudal medulla,28 in the primary somatosensory cortex activation was consistent with the location of the face, and side/back of the head which goes in line with the Penfield homuncular map concept.
Interestingly, in M. Romoli and colleagues in 2014 study's support the auricular points´ specificity and the Penfield homuncular map that the AA explanation relays. The needling of the auricular point found in the Scaphoid fossa, related in AA with the “Thumb” produced an increase in activation bilaterally in the parietal operculum, the region of the secondary somatosensory area. Furthermore, the needling of the auricular point in the antitragus area showed a pattern that essentially corresponds with regions fitting to the pain matrix, with local differences in the left amygdala, anterior cingulate cortex, and cerebellum. The above research provides preliminary evidence that the AA at different points led to other brain structures' activation or deactivation, which seems to overlap regions belonging to the anxiety's neurobiology.69,79 Although most studies have shown several therapeutic effects induced by invasive VN and TN electrical stimulation, those can be reproduced without the electric current.14 However, is still too early to make any recommendations for clinical practice and further randomized trials using fMRI to confirm the AA brain's activation are needed.
Limitations and future challengesThere are several limitations to this study. Firstly, our narrative review offers an overview of the possible mechanisms of AA in anxiety proved by fMRI; however, the used selection of publications is potentially biased since the present study is descriptive but not systematic. One of the primary biases of our work was not to limit the chosen keywords for anxiety. For example, we include an article from Zhang et al. (2019). This published paper reported the application of auricular electrical stimulation in 26 patients with migraines28 (not anxiety). We include this article due to the difficulty of finding papers demonstrating the nuclear magnetic resonance effect of AA in human brains.
Secondly, AA is commonly used in traditional Chinese medicine; the exclusion of Chinese databases and any unpublished trials may impact the results of this narrative review. Finally, only English language publications were retrieved, leaving out potential vital data from other languages. Accordingly, we suggest further updated analysis in the future with the inclusion of Chinese databases and other languages to corroborate this work.
Tendentially, researchers are inclined to confirm the AA effects via the VN and TN stimulation. However, AA covers more areas, calling attention to the possible co-activation of the GAN when triggered through the auricular lobe area. The GAN communicates with several cranial nerves; for example, the anterior branch sends a tiny twig (or several small twigs) into the substance of the parotid gland, connects to the facial nerve,80 and the posterior branch communicates with the auricular branch of the VN.81 So, it would make sense to study the AA effects under GAN stimulation in the future.
Generally, some articles refers to how AA evidence is limited82,83 and unclear8; still, the diversity of AA modalities, such as invasive or noninvasive, may be associated with different treatment effects and physiological mechanisms, even if treating the same clinical condition. Also, the skin is composed of specific sensory receptors (e.g., exteroceptors, interceptors and proprioceptors), which could also be activated by different AA approaches showing supplemental pathways to reach the CNS. In this case, we raise the question if all noninvasive AA techniques performed with seeds, massage, moxibustion, laser light and electrical stimulation, could trigger exteroceptors and proprioceptors. At the same time, the invasive AA (with or without electrostimulation) could also reach interceptors and offer a prompter body response, regardless of noninvasive procedures could be a more practical way to use in young and elderly patients or those who are afraid of needles.84,85 Despite some adverse effects, such as transient dizziness, headaches, and fatigue, have been expressed with noninvasive AA,86 however, these noninvasive techniques also minimize the risk of adverse events, such as infection or bleeding complications.83,86–89
ConclusionInvasive (needling) and non-invasive (electrical stimulation) AA at distinct auricular points led to the activation or deactivation of diverse brain structures. This effect appears to overlap regions belonging to the anxiety's neurobiology.
The attempt to link the AA mechanisms to the cranial nerves combined with an embryological approach is promising. Yet, more studies with imaging and different AA techniques and auricular points are necessary to provide evidence for this effect of AA. Meanwhile, we present a speculatory model in Fig. 7.
Schematic representation of auriculotherapy's possible mechanisms of action. Legend: The trigeminal nerve (TN) sends input to the Central nervous system (CNS) and Thalamus (Th) via the spinal trigeminal nucleus; the vagus nerve (VN) via the solitaries' nucleus tract; and the greater auricular nerve (GAN) via facial and VN. Decreased activation in Locus coeruleus (LC) and in limbic brain areas, including the amygdala (A), hippocampus (Hy), and para-hippocampal gyrus leads to improvements in regulation of cortisol segregation. The increase activation in the parietal operculum and the secondary somatosensory area activation region aligns with the Penfield homuncular map concept. T. The catecholaminergic innervation from the PAG, amygdala, Raphe nucleus (RN) and hypothalamic nuclei towards the solitary nucleus tract supports the mood regulation and the release of serotonin. Schematic illustration created with BioRender.com.
This research received no external funding.
Declaration of Conflicts InterestAV carried out this work as part of her PhD work. There are no other conflicts of interest to declare.
Ethical approvalThis mapping review does not require ethical approval.
CRediT authorship contribution statementAndreia Vieira: Conceptualization, Methodology, Software, Data curation, Writing – original draft. António Moreira: Writing – review & editing. Jorge Machado: Visualization, Supervision.
This article results from work supported by the Abel Salazar Institute of Biomedical Sciences of the University of Porto resources and facilities.