Sperm motility is a crucial factor in male infertility and it depends on mitochondrial tail movements. Photobiomodulation light therapy allows the cells to produce their energy through activation of the mitochondria. The aim of the present study was to examine the impact of photobiomodulation on sperm motility in astenozoospermic individuals.
Materials and methodsFollowing semen analyses of 20 astenozoospermic individuals, collected semen samples were centrifuged. Pellet was obtained and homogenized through mixing with culture media in 1:1 ratio. Each semen samples were divided into 3 groups. In the first group, control samples were not exposed to laser irradiation. The Group 2 and Group 3 were exposed to 650nm wavelength of photobiomodulation from 10cm distance in dark environment via a 36cm2 aperture sizer with 200mW output power for 30 and 60min duration, respectively. Sperm motilities were evaluated and chromatin condensation of sperms was determined.
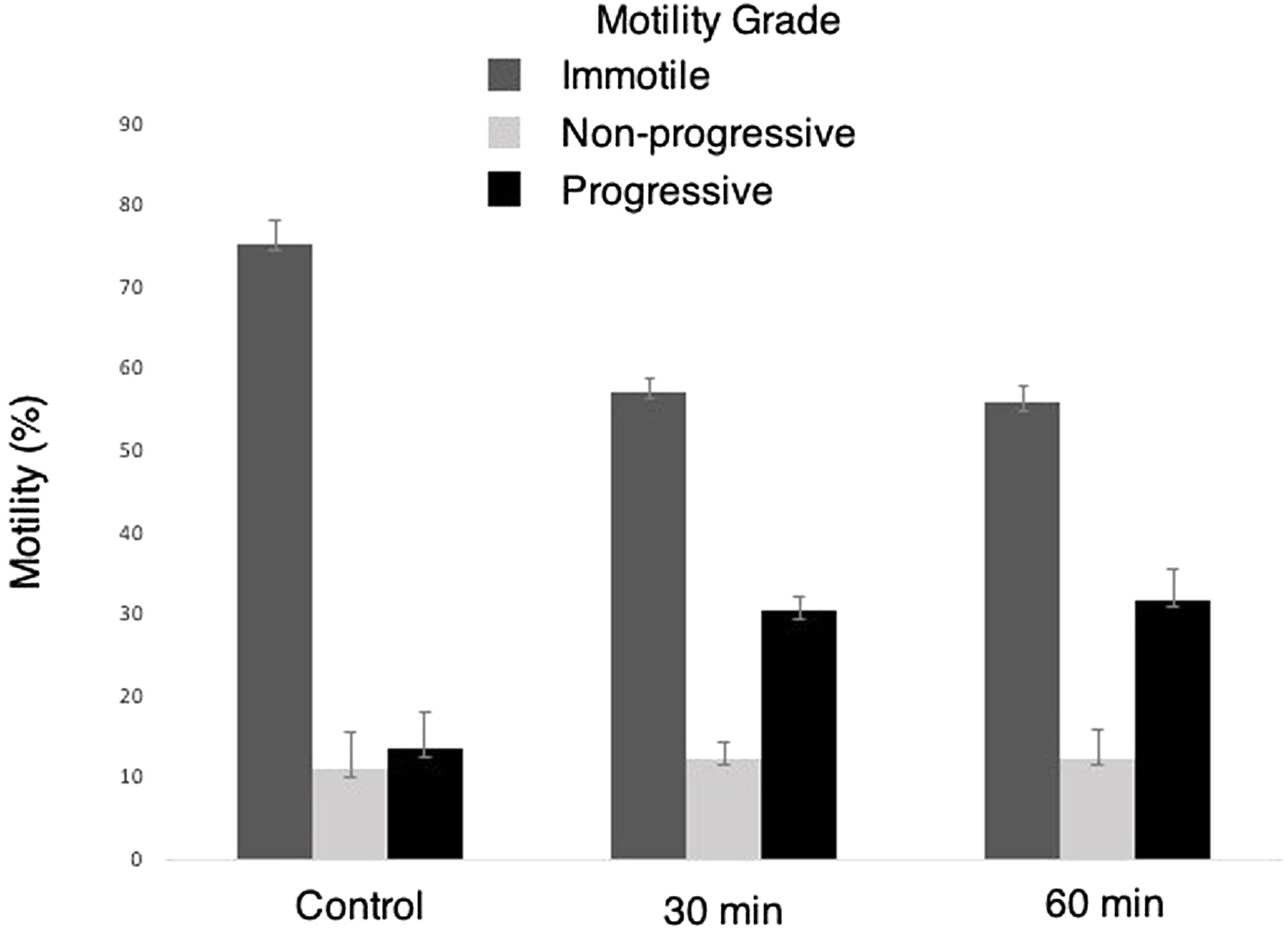
ResultsSperm motilities were significantly increased in photobiomodulation groups compared with the controls. Sperm motilities tended to be different between the 30 and 60min red light exposure groups; however, it was not statistically significant. When the motility grades were compared, no significant difference was observed in non-progressive motility sperms. While immotile sperms decreased significantly in the photobiomodulation groups compared to the control group, progressive sperms increased.
ConclusionsThe results of the present study demonstrated that the photobiomodulation is an efficient method to increase the sperm motility of astenozoospermic individuals independent of the duration of exposure.
La motilidad espermática es un factor crucial en la infertilidad masculina y depende de los movimientos de la cola mitocondrial. La fototerapia de fotobiomodulación permite que las células produzcan su energía a través de la activación de las mitocondrias. El objetivo del presente estudio fue examinar el impacto de la fotobiomodulación en la motilidad de los espermatozoides en individuos astenozoospérmicos.
Materiales y métodosLuego de los análisis de semen de 20 individuos astenozoospérmicos, se centrifugaron las muestras de semen recolectadas. Se obtuvo el sedimento y se homogeneizó mezclándolo con medios de cultivo en una proporción de 1:1. Cada muestra de semen se dividió en 3 grupos. En el primer grupo, las muestras de control no se expusieron a la irradiación láser. El grupo 2 y el grupo 3 fueron expuestos a una longitud de onda de 650nm de fotobiomodulación desde una distancia de 10cm en un ambiente oscuro a través de un medidor de apertura de 36cm2 con una potencia de salida de 200mW durante 30 y 60min de duración, respectivamente. Se evaluaron las motilidades de los espermatozoides y se determinó el tamaño de la cromatina de los espermatozoides.
ResultadosLa motilidad de los espermatozoides aumentó significativamente en los grupos de fotobiomodulación en comparación con los controles. La motilidad de los espermatozoides tendió a ser diferente entre los grupos de exposición a la luz roja de 30 y 60min; sin embargo, no fue estadísticamente significativo. Cuando se compararon los grados de motilidad, no se observaron diferencias significativas en los espermatozoides de motilidad no progresiva. Mientras que los espermatozoides inmóviles disminuyeron significativamente en los grupos de fotobiomodulación en comparación con el grupo de control, los espermatozoides progresivos aumentaron.
ConclusionesLos resultados del presente estudio demostraron que la fotobiomodulación es un método eficiente para aumentar la motilidad de los espermatozoides de los individuos astenozoospérmicos independientemente de la duración de la exposición.
Approximately 30–50% of all infertility cases are caused by male related factors.1,2 Fertilization ratios and embryonic development depends on sperm associated problems, including sperm concentration, motility and viability.3,4 Reduced sperm concentration and motility in infertile males is usually associated with abnormal sperm morphology and damaged deoxyribonucleic acid (DNA).2,5,6 Abnormal sperm morphology as well as decreased number and motility may reduce the chance of natural conception. Therefore, assisted reproductive techniques (ART) provide various solutions for infertile couples.5
Elevated demand of ART has been resulted in increased requirements to improve the routine practices in IVF laboratories. Preparation of semen samples is one of the significant concerns in in vitro fertilization (IVF).7 Sperm preparation is the first step in ART. The main aim in this procedure is the selection of a highly motile normospermic sperms with minimal DNA damage.8–11
Motility is one of the most important characteristic features of the sperm's ability to fertilize and it is positively associated with energy consumption. Moreover, motility is also associated with the sperm viability and morphological integrity.1,12,13 Mitochondria have a key role in normal sperm functions. In addition to the formation of intracellular Ca2+ and NO, they are also important for an increased amount of adenosine triphosphate (ATP), sperm movement, capacitation and the control of acrosome reactions.14
Low-level light therapy (LLLT), or recently adopted photobiomodulation therapy (PBMT), is typically the application of light, at approximately 600–1000nm wavelength range, in order to directly stimulate or inhibit cellular and biological processes.14,15 Generation of the biological effects of PBMT is based on the propagation of electromagnetic waves of a laser optical system interacting with cells and tissues. Photo-receptors that absorb electromagnetic energy mediate these biological effects. Moreover, irradiation parameters and target cell type are also important for mediating the effects.3
The photo-stimulatory effects of photobiomodulation on somatic cells, including fibroblast proliferation, reduced cell death, stimulation of osteoblast activity and bone development, and increased phagocytic activity of monocytes, has been studied extensively.12 Numerous beneficial effects of LLLT has been reported in previously published studies, including improved tissue healing after trauma, increased collagen production, promoted neural growth, advanced DNA and RNA synthesis, increased cell adhesion as well as retinoprotective and post-ischemia protective effects on cardiomyocytes. Moreover, improved healing of muscoskeletal injuries, dermal diseases, degenerative diseases, neuropathic pain syndromes, and even traumatic brain injuries as well as reduced inflammation and edema and recovery of blood flow has been previously reported following LLLT therapies.1,3,16–18
Biological mechanisms of photobiomodulation have not been fully understood yet. However, some studies suggested that absorption of light through primary endogenous chromophores, such as mitochondrial enzymes, might be involved in the process.12 A single cell is composed of several components that contain chromophores. Mitochondria, which has the highest intracellular chromophore concentration, might be one of the most important components inside the cell, especially the ones containing cytochrome of the electron transport chain.1 Moreover, activation of specific receptors or messengers at the molecular level could explain the biological response following photobiomodulation. Some studies suggested the impact of secondary messengers, including cAMP, Ca2+, and reactive oxygen species (ROS) on sperm motility. Biostimulation of isolated mitochondria through laser irradiation resulted in the activation of respiratory chain14 and increased ATP synthesis.4,16
Mitochondrial dysfunctions or structural defects at the tail disrupt sperm motility in astenozoospermia. Sperm motility might be increased following PBMT in astenozoospermic individuals through increased mitochondrial functions in different cells and tissues.3,14 Less is known about the photo-stimulatory effects of PBMT on human sperms. The aim of the present study was to evaluate the impact of 650nm wavelength laser radiation at different durations on low motility human sperms.
Material and methodsThe current study was performed on waste sperm specimens obtained from males with no history of cancer or chronic disease between 25 and 40 years of age, who admitted to Bolu Abant Izzet Baysal University College of Medicine for semen analyses. All participants provided the informed constent form. The present study was carried out by 2018/107 numbered local ethical committee decision. 20 astenozoospermic individual were included in the study, who met the 2010 critieria of the World Health Organization (WHO) (Table 1). Astenozoospermia were described by the WHO based on the 2010 criteria as; ≤40% sperm motility.19 No pathology (bacterial infection, hormonal disorder, trauma, etc.). Semen samples were destroyed at the termination of study.
The values of mean semen analysis of patients according to the criteria of the World Health Organization (WHO 2010).
| Seminal characteristics | WHO 2010 | Semen analysis of patients |
|---|---|---|
| Volume (ml) | 1.5 (1.4–1.7) | 2.7 |
| Spermatozoa concentration (106/ml) | 15 (12–16) | 42 |
| Total spermatozoa concentration (106/ml) | 39 (33–46) | 71.4 |
| Total motility (PR+NP, %) | 40 (38–42) | 24.65 |
| Morphology (normal forms, %) | 4 (3.0–4.0) | 8.7 |
According to WHO 2010 criteria, semen samples obtained from 20 patients by masturbation following 3 days of sexual abstinence. Samples were liquefied by keeping them in non-toxic polypropylene sterile storage containers for 25min. Then, physical evaluation of samples was performed by assessing color, odor, viscosity, and volume. Microscopic evaluation of the sperm concentration and motility were performed using Makler counting chamber (Sefi Medical Instruments) under Nikon T1A input AC light microscope. Sperm were stained by Diff-quick method for morphological evaluation.
Preparation of the semen samplesAfter the semen analyses, excess samples from each participant were centrifuged in conical tubes (falcon, Lot: 352092) at 1000rpm for a 15min duration. Then, the supernatant (seminal plasma) was removed. The pellet was homogenized in a 1:1 ratio by using Sperm Rinse Medium (Vitrolife, Gothenburg, Sweden, Lot: 507.371). The resulting homogenate were prepared for photobiomodulation by equally dividing the sample into two sterile eppendorf tubes, each containing 1.5ml of volume (Slab, Cat No: L7290).
Sperm photobiomodulationRed-light penetrates the mitochondria through increasing the permeability of semi-permeable cell membrane. As a result, it increases the ATP production and transmits the proton therapy resonance into the cell. The light source of the present study, which was used to induce photobiomodulation, was a B.E.A.T Light Rent (BIREGS-Germany, SN:C10991) Low Level Laser Therapy. The LLLT device irradiates from 36cm2 aperture size at a 650nm wavelength with 200mW output power. The semen samples in eppendorf tubes were exposed to LLLT in CO2 incubators from 10cm distance for 30 and 60min duration in a dark environment at 35°C temperature (Fig. 1).
Evaluation of sperm motilityFollowing a LLLT exposure for 30 and 60min, motility of semen samples was reevaluated using Makler counting chamber (Sefi Medical Instruments) under Nikon T1A input AC light microscope. According to the WHO 2010 criteria,19 sperms were evaluated as progressive (PR), non-progressive (NP) and immotile. Since semen samples from each individual were not exposed to red-light simultaneously, each sample were compared with its own control group (prior to LLLT) (video).
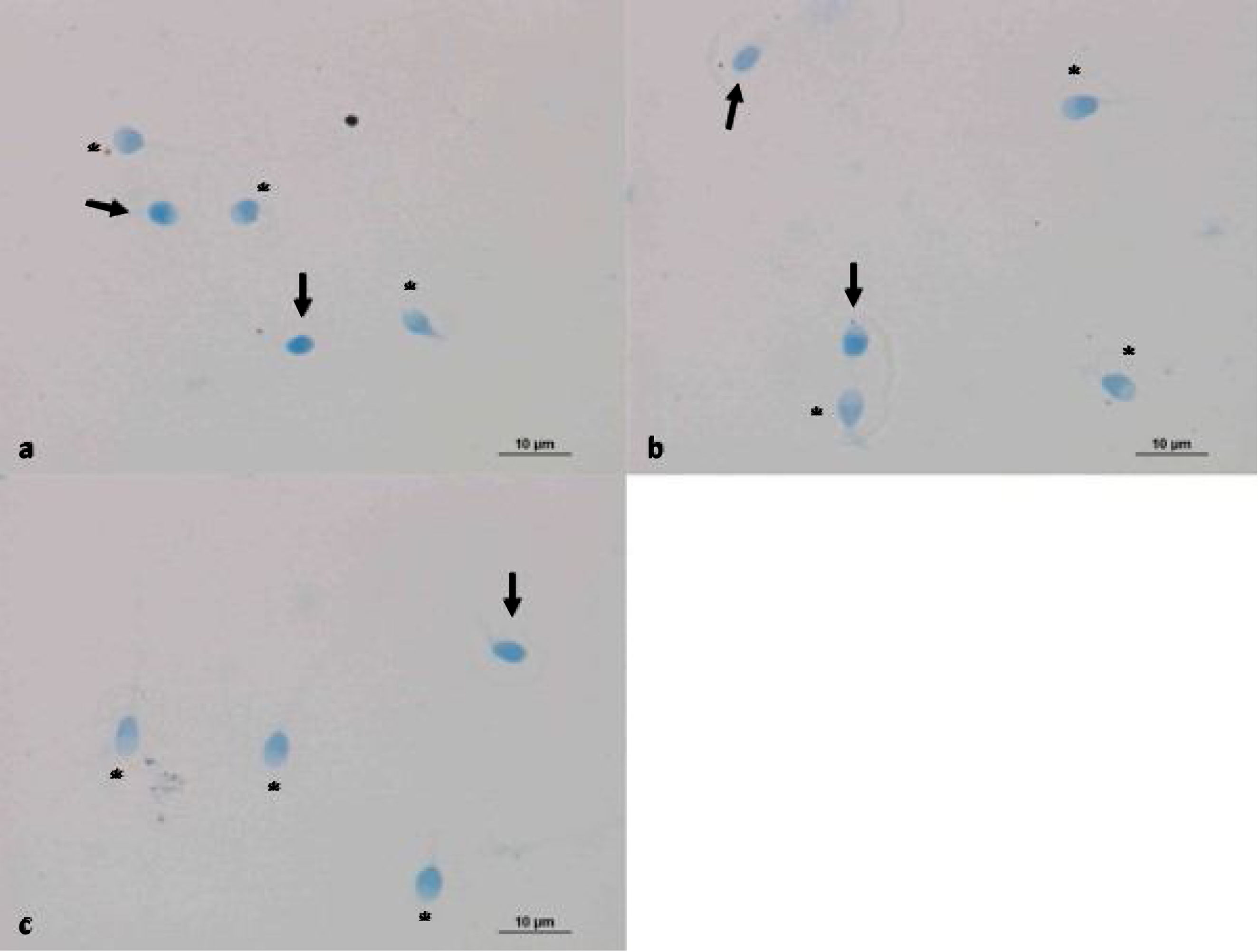
Evaluation of sperm condensation through aniline blue stainingAniline blue is a frequently used stain to assess the chromatin integrity or maturation of sperm cells. The nucleus of sperm cells with damaged DNA shows positive reactions with aniline blue staining. However, healthy sperm nucleus did not react with the dye and thus were not stained.20 Aniline blue staining was performed after the light exposure to confirm the reliability of photobiomodulation. Smear samples were prepared on poly-lysine slides by obtaining 10μl homogenates from both controls and 30 and 60min red-light exposed group for the evaluation of chromatin integrity. Air-dried smear samples were fixed in 0.2M PBS (Phosphate Buffered Saline, Thermo Fisher Scientific, UK pH 7.2 Lot: mk170117) containing 3% glutaraldehyde for 30min. Then, the samples were placed in 5% aqueous aniline blue stain (Carlo Erba, cat no: 428582, France) containing 4% acetic acid (pH 3.5) for 5–8min. Samples were washed in PBS twice and a cover slip were applied for microscopic examination (Nikon Eclipse 80i light microscope). 200 cells were counted with 40× magnification objectives. Chromatin condensation defects of 3 groups were compared. Sperm cells that absorbed blue stain were considered as having a damaged DNA.
Statistical analysesA repeated measure ANOVA was performed to compare the averages of data obtained from different groups and measurement time points. Sphericity assumption was assessed with Mauchly's test. Post hoc Bonferroni test was used to perform multiple group comparisons. Motility percentages were summarized with mean±SEM. Differences between groups in groups that did not show normal distribution were measured using the non-parametric Kruskal–Wallis test. Tamhane's T2 was applied as a posthoc test. Significance was set at p<0.05 and the analyses were performed using the Statistical Package for Social Sciences 25.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
ResultsThe present study was performed on sperms obtained from individuals, who admitted to Bolu Abant Izzet Baysal University College of Medicine for semen analyses. Astenozoospermic speciemens were included into the study throughout rutine semen analyses based on WHO 2010 criteria. The motility of 30 and 60min groups exposed to red light were evaluated using Makler chambers. Besides the motility of control group that was not exposed to red light was evaluated using Makler chambers. Following the motility assessments, chromatin integrity was evaluated with 10μl of smear samples stained with aniline blue.
Motility of sperms exposed to 30 and 60min red-light were significantly increased compared to the own control groups. These results demonstrated the activation of mitochondria through red-light (Fig. 2).
Repeated measures ANOVA with a Greenhouse–Geisser correction determined that the mean motility rate (%) was significantly different in 30min and 60min Groups (p<0.001; Table 2).
Evaluation of the time effects on sperm motility grades suggested that following the 30min and 60min light exposure there was a significantly decreased number of immotile sperms when compared to the own control groups. On the other hand, even though the number of non-progressive sperm motility tended to increase after LLLT exposure, it was not statistically significant. LLLT exposure significantly increased the number of progressive motile sperms. Even though all motility grades demonstrated a different motility tendency between 30 and 60min exposure groups, the results were not statistically significant. These results demonstrated a time associated increase in the motility of sperms following red-light exposure. However, a statistically significant difference was not observed between the exposure periods tested in the present study (Table 3).
Time associated evaluation of sperm motility grades.
| Multiple comparisons | |||||||
|---|---|---|---|---|---|---|---|
| Bonferroni | |||||||
| Dependent variable | (I)Group | (J)Group | Mean difference (I−J) | Std. error | Sig.b | 95% confidence interval | |
| Lower bound | Upper bound | ||||||
| Control | Immotil | NP | 64.250* | 2.969 | .000 | 56.93 | 71.57 |
| PR | 61.800* | 2.969 | .000 | 54.48 | 69.12 | ||
| NP | Immotil | −64.250* | 2.969 | .000 | −71.57 | −56.93 | |
| PR | −2.450 | 2.969 | 1.000 | −9.77 | 4.87 | ||
| PR | Immotil | −61.800* | 2.969 | .000 | −69.12 | −54.48 | |
| NP | 2.450 | 2.969 | 1.000 | −4.87 | 9.77 | ||
| 30min | Immotil | NP | 44.800* | 4.880 | .000 | 32.76 | 56.84 |
| PR | 26.950* | 4.880 | .000 | 14.91 | 38.99 | ||
| NP | Immotil | −44.800* | 4.880 | .000 | −56.84 | −32.76 | |
| PR | −17.850* | 4.880 | .002 | −29.89 | −5.81 | ||
| PR | Immotil | −26.950* | 4.880 | .000 | −38.99 | −14.91 | |
| NP | 17.850* | 4.880 | .002 | 5.81 | 29.89 | ||
| 60min | Immotil | NP | 43.450* | 4.954 | .000 | 31.23 | 55.67 |
| PR | 24.150* | 4.954 | .000 | 11.93 | 36.37 | ||
| NP | Immotil | −43.450* | 4.954 | .000 | −55.67 | −31.23 | |
| PR | −19.300* | 4.954 | .001 | −31.52 | −7.08 | ||
| PR | Immotil | −24.150* | 4.954 | .000 | −36.37 | −11.93 | |
| NP | 19.300* | 4.954 | .001 | 7.08 | 31.52 | ||
The reliability of photobiomodulation was evaluated via aniline blue staining in control samples and sperms exposed to 30 and 60min LLLT exposure (Fig. 3). No significant difference was found between the groups (Table 4).
Time associated evaluation of sperm chromatin condensation.
| Multiple comparisons | ||||||
|---|---|---|---|---|---|---|
| (I)Groups | (J)Groups | Mean difference (I−J) | Std. error | Sig. | 95% confidence interval | |
| Lower bound | Upper bound | |||||
| Control | 30min | .300 | 2.389 | .999 | −5.67 | 6.27 |
| 60min | −.650 | 2.487 | .991 | −6.86 | 5.56 | |
| 30min | Control | −.300 | 2.389 | .999 | −6.27 | 5.67 |
| 60min | −.950 | 2.418 | .972 | −6.99 | 5.09 | |
| 60min | Control | .650 | 2.487 | .991 | −5.56 | 6.86 |
| 30min | .950 | 2.418 | .972 | −5.09 | ||
Fertilization and embryonic development depends on sperm factors, including motility and viability.3 Only a few of the sperms ejaculated inside of vagina are able to reach the oocyte. This is a natural sperm selection process occurring in female gential track.2 Sperm motility is critically important for the transport of male nucleus to the oocyte during fertilization. Therefore, sperm motility is required to reach the oocyte. This process is carried out with a mitochondrion that is considered an organelle required for the tail movement.12 A mature human sperm is composed of a head carrying the genetic material, neck (centriole), and mid-piece and tail containing numerous mitochondria. Mitochondrion is an organelle that produces the energy required for the movement of sperm tail. Structural tail defects or mitochondrial malformations are responsible for the disrupted sperm motility in astenozoospermic individuals.14 Today, LLLT or PBMT are frequently used in different fields of medical treatment. PBMT treatment is able to repair mitochondrial functions in various cells and tissues. Consequently, sperm motility could be improved following a PBMT in astenozoospermic individuals. Therefore, in the present study, the effects of laser irradiation on human sperm motility were investigated.
In the current study, application of LLLT, which irradiates from 36cm2 aperture size at a 650nm wavelength with 200mW output power, significantly increased the sperm motility. This data supported the previous findings for the chemical mechanism of intracellular photonic absorption. Moreover, the results of the present study also suggested that the photonic energy in the red light was emitted by cytochrome-c oxidase, causing an increased ATP production. As a result, sperm motility was increased.21 Previously published studies demonstrated that the beneficial effects of LLLT were achieved through increased mitochondrial respiration and ATP synthesis.14
A large number of studies have been conducted in different animal models regarding the effect of red light on sperm cells. Studies in other mammalian species have shown that stimulation of semen with red light increases sperm motility, mitochondrial activity, and fertilization capacity.22 Catalan et al. investigated how donkey and horse sperm cells stimulated by red light affect mitochondrial function. With their findings, they proved that red light increases sperm motility.22,23 In a study on bull sperm, Sharifzadeh et al. exposed sperm samples to photobiomodulation and observed that motility improved. They suggested that this result may increase fertilization.24 In another study, Rezaei et al. observed that photobiomodulation treatment increased mice sperm motility and decreased the number of apoptotic cells.25 Our study has shown that photobiomodulation increases sperm motility in line with these studies. In addition, the absence of an increase in the number of damaged cells proved that red light does not have a possible harmful effect on cells.
Moreover, Cohen et al. demonstrated an increased fertility potential of mouse sperms following an administration of laser treatment at 630nm wavelength.26 Ocana-Quero et al. investigated the biological impacts of He–Ne laser on the acrosome reactions of bull sperm cells.27 Iaffaldano et al. showed an increased motility of freeze-thawed turkey sperms following a laser treatment at doses of 3.24–5.4J/cm.2,28 Moreover, Iaffaldano et al. also demonstrated that the laser irradiation in freeze-thawed rabbit sperm protected mitochondrial activity and prevented freezing associated damages.29 Rivera et al. exposed fresh dog sperms to various doses (0, 4, 6 and 10J/cm2) and durations (0–15–45min) of laser irradiation at 655nm wavelength. Their results showed an increased sperm motility after 45min exposure.30 Likewise, in the current study, increased sperm motility was observed following an exposure to 650nm wavelength laser irradiation.
Some studies have suggested that red light's increase in sperm motility is time dependent.31–33 Yazdi et al. demonstrated that 45 and 60min exposure of human sperms to laser irradiation at 830nm wavelength resulted in increased sperm motility.12 The results obtained in the present study were comparable with that of Yazdi et al. In a study of Helena Ban Frangez et al. exposure of astenozoospermic sperms to laser irradiation for 3min increased the motility and that it was independent of the administered wavelength.14 In the current study, increased sperm motility was also observed following 30min of exposure. Frestone et al. observed a significant increase in sperm motility following an exposure of red-light for 30min to 33 specimens.34 However, Crespo et al. exposed porcine sperm to photobiomodulation for 10min and did not observe any difference in sperm motility and fertilization.35 In the study of Salama and El-Sawy, 2, 5 and 10min exposure of 27 astenozoospermic and non-astenozoospermic sperms to red-light significantly increased the progressive sperm motility. No significant difference was found in aniline blue and HOS tests.1 Siqueira et al. exposed cattle sperms to laser irradiations for 5 and 10min. Their results suggested that 5min exposure of laser irradiation did not have any significant effects. However, 30min incubation of sperms following 10min of laser exposure significantly increased the motility.3 The results of the present study also supported these findings.
ConclusionIn summary, laser irradiation has been used for preimplantation genetic diagnosis in ART. The results of the current study demonstrated an increased motility of astenozoospermic individuals following photobiomodulation light therapy. These findings are promising. Given the reliability of this new methodology, further research is required to prevent potential chromatin and DNA damage to human sperms before the start of its clinical practice.
Authors’ contributionsAS, TF, OMY: participated in study design, data collection and evaluation, drafting and statistical analysis. AS and TF: performed experimental procedure. TF and OMY contributed extensively in interpretation of the data and the conclusion. All authors performed editing and approving the final version of this paper for submission, also participated in the finalization of the manuscript and approved the final draft.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis study has been funded by Bolu Abant Izzet Baysal University Scientific Research Project (Code: 2018.08.03.1390).
Conflict of interestThe authors declare that there are no conflicts of interest.
The authors would like to thank to everyone who contributed the present study, including Gizem Dede, a master's student in the histology-embryology department and Burak Serefli, andrology laboratory technician. A special thank you goes out to the sales manager of Frequences Ilo, Damien Roy, for providing the technical and physical information of the redlight device used in the study.