Human semen analysis must be performed after the liquefaction of the ejaculate. This takes place about 30min after ejaculation and samples must be maintained in the lab during this time. The temperatures for this incubation and the final analysis of motility are crucial but seldom taken into account. This study aims to examine the effect of these temperatures on various sperm parameters both manually (sperm count, motility, morphology, viability, chromatin condensation and maturation and DNA fragmentation) and CASA (kinematics and morphometrics, using an ISAS®v1 CASA-Mot and CASA-Morph systems, respectively) analyzed.
MethodsSeminal samples from thirteen donors were incubated for 10min at 37°C followed by additional 20min at either room temperature (RT, 23°C) or 37°C and then examined following WHO 2010 criteria.
ResultsThe data obtained show that there were no significant differences (P>0.05) in the subjective sperm quality parameters with incubation temperature. On the other hand, the head sperm morphometric parameters were significantly higher after room temperature incubation showing, in addition, lower ellipticity (P<0.05). Furthermore, kinematic parameters were evaluated both at RT and 37°C for the two incubation temperatures. In general, the four temperature combinations showed that kinematic parameters followed this order: RT-RT
ConclusionsOur results showed that temperature control during both incubation and analysis is needed for accurate semen analysis, recommending the use of 37°C during the entire process.
El análisis de semen humano debe realizarse después de la licuefacción del eyaculado. Esto ocurre aproximadamente a los 30minutos después de la eyaculación. Las temperaturas para esta incubación y el análisis final de la motilidad son cruciales, pero rara vez se tienen en cuenta. Este estudio tiene como objetivo examinar el efecto de estas temperaturas en varios parámetros de los espermatozoides tanto de forma manual (recuento de espermatozoides, motilidad, morfología, viabilidad, condensación y maduración de la cromatina y fragmentación del ADN) como CASA (cinemática y morfometría, utilizando un CASA-Mot ISAS®v1 y Sistemas CASA-Morph, respectivamente) analizados.
MétodosLas muestras seminales de 13 donantes se incubaron durante 10minutos a 37°C, seguidas de 20minutos adicionales a temperatura ambiente (TA, 23°C) o a 37°C y luego se examinaron siguiendo los criterios de la OMS 2010.
ResultadosLos datos obtenidos muestran que no hubo diferencias significativas (p>0,05) en los parámetros subjetivos de calidad del esperma con la temperatura de incubación. Por otro lado, los parámetros morfométricos de la cabeza de los espermatozoides fueron significativamente más altos después de la incubación a temperatura ambiente, mostrando, además, una elipticidad más baja (p<0,05). Además, los parámetros cinemáticos se evaluaron tanto a temperatura ambiente como a 37°C para las dos temperaturas de incubación. En general, las cuatro combinaciones de temperatura mostraron que los parámetros cinemáticos siguieron este orden: RT-RT < RT-37 < 37-37 < 37-RT (temperaturas de incubación y análisis, respectivamente).
ConclusionesNuestros resultados mostraron que el control de la temperatura durante la incubación y el análisis es necesario para un análisis de semen preciso, recomendando el uso de 37°C durante todo el proceso.
Human fertility has been matter of concern for our species since Egyptian times, about 2000BCE.24 Time was needed to the first observation of spermatozoa on 1677, when Antonie Philips van Leeuwenhoek discovered them and termed them animalcules.38 Since then the study of sperm function and characteristics has been related to the resolution of fertility problems.30 The arrival of the assisted reproduction started with artificial insemination as early as 1770s by John Hunter in humans27 and Lazzaro Spallanzani in dog.16,32 But only in the decade of 1920 artificial insemination was extended like a common procedure in farm animal reproduction.15 It was in the 1940s when artificial insemination became usual employing homologous semen and later introducing donor samples for this purpose.27 With the advent of IVF in 1978 a new era in human assisted revolution started. The first human pregnancy using external fertilization achieved by Edwards, Steptoe and Purdy opened the door to new approaches and solutions for remedying infertility.7,8 Finally, Palermo's team was able to perform the first successful ICSI, allowing men with severe subfertility to conceive a baby.28 Additional significant achievements were obtained in recent times, particularly related with cryopreservation and selection of the potentially optimal spermatozoa.27
Most procedures of assisted reproduction begin with a semen analysis, meant to evaluate the potential fertilizing ability of an ejaculate from a given male. Sperm quality varies depending on both biological and technical variables. Obviously, the objective of the semen analysis is to evaluate the biological condition of the sample, but this is not possible if a correct and standard method is not applied.6 Among technical limitations for a reliable assessment it is important to define how and where the sample is collected and maintained before analysis and the analytical methods used. To achieve this goal, WHO has published several manuals from 1980 to 2010 when the fifth edition was published.39
Human samples must be analyzed after liquefaction which takes place, under normal conditions, in about 30min after sample collection. The WHO recommends maintaining the samples at 37°C during this time, although if this is not possible, when sampling is done at home, for instance, 20°C is also accepted. The same recommendation exists for motility analysis, with an optimal temperature that provided by a heated microscope stage (at 37°C) but it is also acceptable to perform the analysis at room temperature (RT), usually 22–23°C. The main recommendation is to standardize the procedure for each laboratory.39
Nevertheless, it is known that one major factor affecting semen quality, particularly sperm motility, is temperature. In general terms, spermatozoa of mammals are very sensitive to temperature fluctuations22 with species and individual differences. Spermatozoa from some species, such as the boar, are particularly susceptible to cold shock, especially when stored below 15°C. Sperm motility is better in undiluted semen samples stored at 15°C and 20°C for 48h compared to 4°C and 39°C.41 Equine semen should be kept at 37°C prior to dilution.17 In the case of goat, semen is usually preserved at 18–22°C or at 37°C until adding an extender.2
In the case of human samples, there is little evidence for the effect of incubation and analysis temperatures on sperm quality. In general, it is accepted that incubation at 37°C renders better results than room temperature,10 at least for short incubation times. But when longer time is required the evidence is that room temperature seems to preserve better semen quality.21
Biological measurements are done to define biological variability among individuals, in our case to predict fertility from semen analysis. But any measurement technique includes also another variation source named technical variability. In order to compare results and to increase the biological meaning of the measurements it is needed to standardize the methods as much as possible. This was the aim of WHO when publishing its manuals for semen analysis.39 The aim of the present work was to compare sperm kinematics and morphometry in two incubation and analysis temperatures: RT (23°C) and 37°C, to define the optimal procedure to standardize the routine human semen analysis.
Materials and methodsSamplesIndividual ejaculates from 13 adult normozoospermic volunteers (aged 26±9, range 19–53) were used in the study, after signing a consent report indicating the samples were used exclusively for the purpose of the study and being destroyed after its use. Ethical committee from IVI Valencia EC (1705-UV-035-AG) was obtained.
All the samples were obtained by masturbation, under the same conditions in laboratory facilities, in a sterile receptacle and after 2–5 days of ejaculatory abstinence period. The receptacle used contained chymotrypsin to accelerate the liquefaction time homogenizing the manipulation process of all the samples.
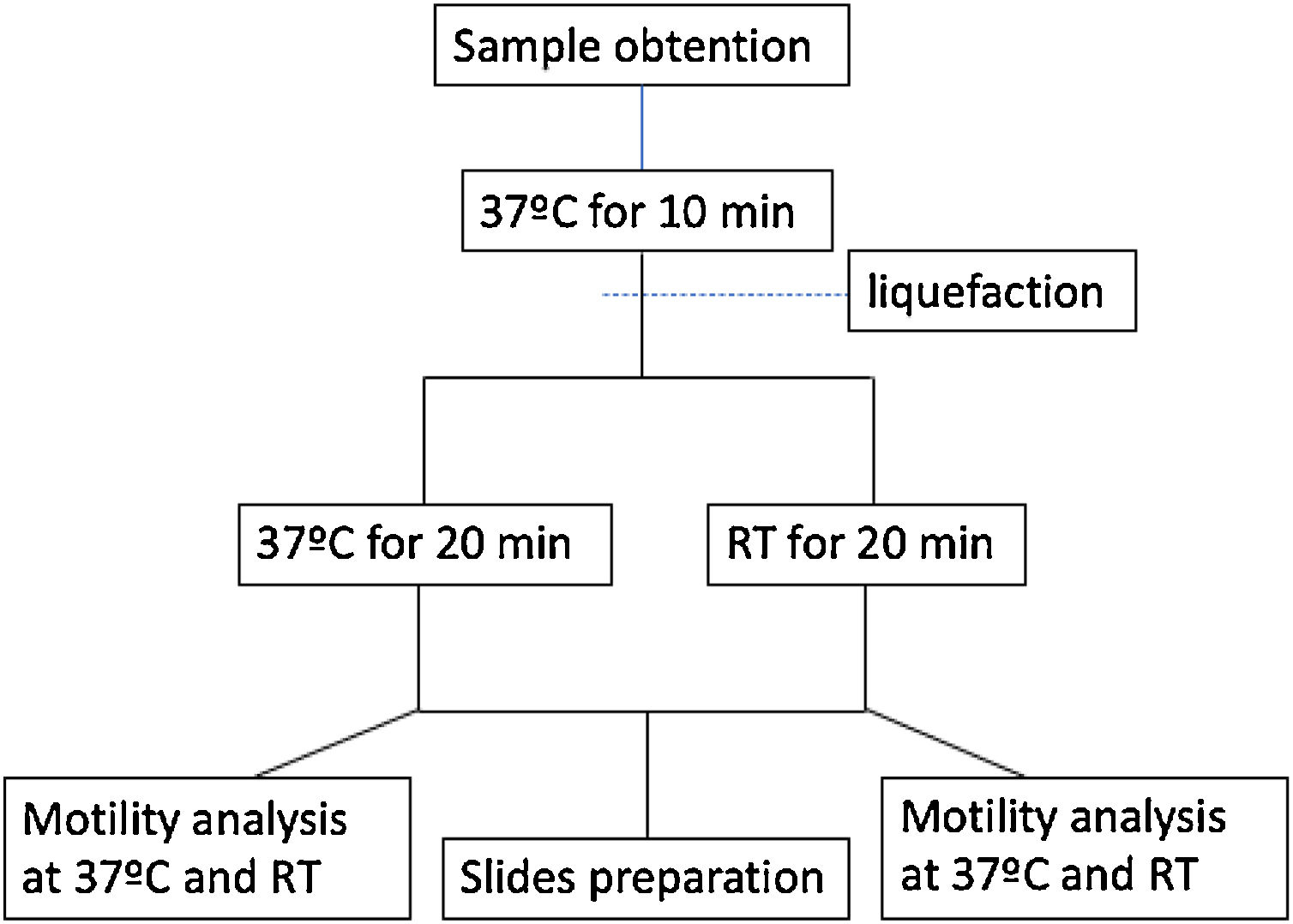
Experimental designTo ensure complete liquefaction, all the samples were incubated for 10min at 37°C. After this time samples were divided in two portions, one incubated at 37°C and another at RT for additional 20min. During this time samples were protected from light exposure (Fig. 1).
Slides were then prepared for morphology, viability, DNA fragmentation, chromatin maturation and chromatin condensation (see below). In addition, motility analyses were performed at both 37°C and RT on a heated microscope stage (Fig. 1).
All the probes were made after well mixing the samples just before taking every drop for every analysis. For each analysis at least 200cells were evaluated on each sample.
Total sperm countSperm concentration was assessed following39 indications. Briefly, an estimation of concentration was obtained during kinematic analysis with a CASA-Mot system (see below) and diluted with formalin solution depending on estimated concentration. With this solution a Neubauer improved counting chamber was loaded and at least 200cells were counted under a negative phase-contras microscope at ×200 magnification. Total count was obtained multiplying the concentration (106/mL) by the volume of each sample like a most representative counting value.
Sperm motility and kinematicsBoth motility and kinematics evaluation were done using an ISAS®v1 CASA-Mot system (Proiser R+D, S.L., Paterna, Spain), including a video camera ISAS® CM13-ON mounted on a UB203 (OUP/Proiser) negative phase-contrast microscope with a heated stage (37±1°C), using a 10x objective (NA 0.25). The array size of the video frame grabber was 648×488×8 bits and 256 grey levels. Resolution of images was 0.70μm per pixel in both the horizontal and vertical axes. The tail detection facility of the system was activated for ignoring non-sperm particles, with a particle area between 5 and 80μm2 and a connectivity value of 10μm. Track recognition mistakes were deleted, when needed, to avoid the introduction of distortions in the final results.
A Spermtrack® (Proiser) reusable counting chamber of 10μm depth was filled with 2.5μL of raw sample and used on the CASA system to capture videos of 1s at a frame rate of 50 fps on nine fields distributed along the counting area. When required, the chamber was previously heated to 37°C before use.
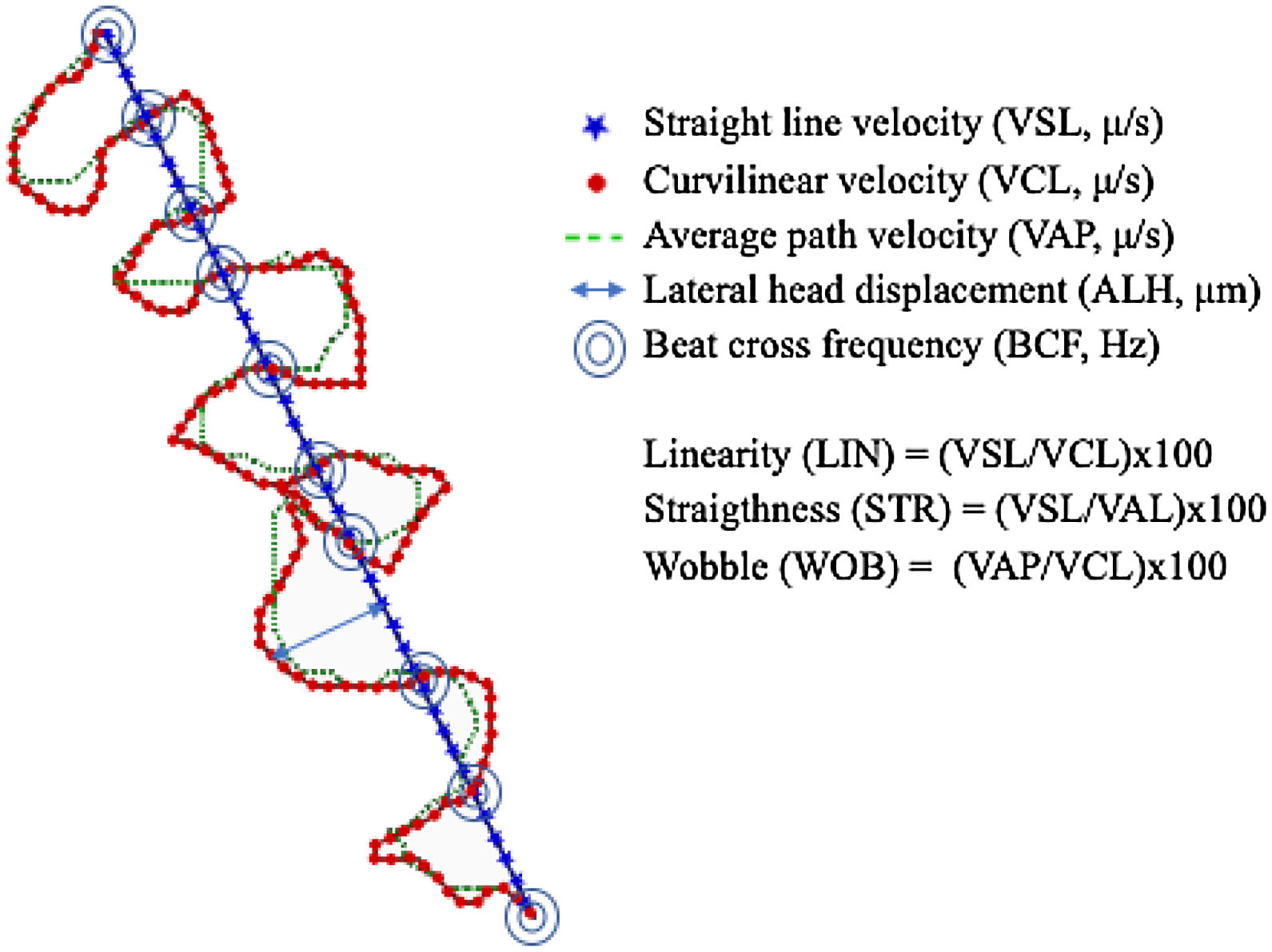
Total and progressive motility were automatically analyzed by the system. In addition, seven kinematic parameters (Fig. 2) were calculated in the four different experimental conditions of incubation and analysis temperature: RT-RT, RT-37, 37-37, and 37-RT.
Sperm morphology and morphometricsTwo preparations for each sample were made by smearing 5μL of semen on clean slides, air drying for 30min and staining using the Diff-Quick Fast Panoptic kit (Medion Diagnostics, Düdingen, Sitzerland). The immersion time on each of the three solutions was 1min, and slides were mounted following the previous described procedure. Normal morphology was evaluated following the criteria of Ref.39
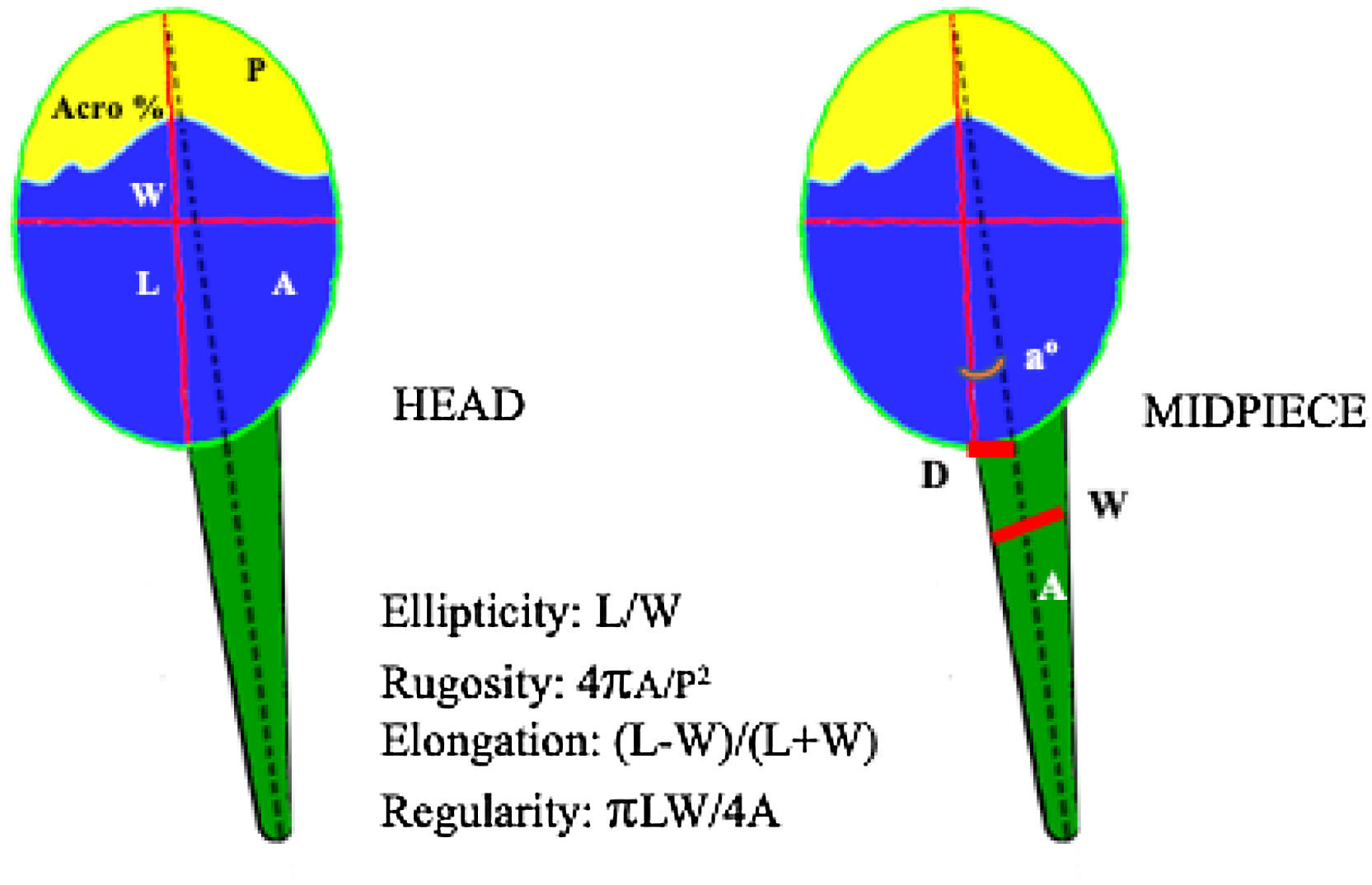
Additionally, ISAS®v1 CASA-Morph system was used for morphometric analysis. The general characteristics of the system were as described above, but in this case a brightfield 40x objective (NA0.70) was used without any filter on the condenser for a final resolution of 0.1215μm/pixel side. Spermatozoa that appeared aggregated or with crossing tails were excluded of the analysis. A total of 13 morphometric values were obtained for both head and midpiece (Fig. 3).
Morphometric parameters analyzed by ISAS®v1 CASA-Morph system. Head: A, area (μm2); P, perimeter (μm); L, length (μm); W, width (μm); Acro, acrosome area (% of the head); Midpiece: a, angle between head and midpiece axis (°); D, distance between the tangential point of the head and midpiece (μm); W, maximum width (μm); A, area (μm2).
In an eppendorf tube, 10μL of sample were well mixed with 20μL of eosin and 10μL of nigrosine (Merck KGaA., Darmstadt, Germany) and 5μL of this suspension were placed and smeared on a clean slide and air dried for 30min. After this time preparations were mounted by immersion for 1s in a solution of Neo-Clear® (Merck, KGaA), placing one drop of Neo-Mount® (Merck KGaA) and one coverslip. Analysis considered white cells as viable and pink ones as non-viable.
DNA fragmentationFor this analysis Halosperm® G2 kit (Halotech DNA, Madrid, Spain) was used following the protocol indicated by the manufacturer. Big and medium halo were considered not fragmented while small and no halo were evaluated as fragmented spermatozoa.
Chromatin maturation and condensationFor maturation analysis, smears on clean slides were fixed with formalin at 4% (Probus, S.A., Badalona, Spain) for 5min, cleaned with distilled water and stained with Aniline blue 5% (Merck KGaA) for 5min, and mounted following the common protocol.
Condensation level of sperm chromatin was evaluated using the SCMA kit (Avicena Research Institute, Teheran, Iran). After preparing the smears on clean slides, they were immersed in solution A at 4°C for 30min, in solution B, at RT for 7min, and in solution C at RT for 3min. Slides were rinsed with distilled water and mounted as before.
Statistical analysisThe data obtained from the evaluations of all ejaculates and fertility were analyzed by descriptive statistics. Distribution properties for all variables were also explored using histograms and probability plots to check for a normal distribution. The analysis of variance was further applied to evaluate statistical differences between temperatures of analysis on seminal parameters. Furthermore, the effect of the incubation was analyzed, also by analysis of the variance, for all kinematic and morphometric variables. The statistical model used was:
where Xijk=measured sperm kinematic variable; μ=overall mean of variable x; Ti=effect of temperature Ij=effect of incubation period; TI(ij)=effect of interaction between temperature*incubation period; ɛijk=residual.The threshold for significance was defined at P<0.05. Further, pairwise comparison between means effects of incubation and temperature were performed by the Tukey–Kramer test. Results were presented as mean±standard deviation of the mean. All data were analyzed using the IBM SPSS package, version 23.0 for Windows (SPSS Inc., Chicago, IL, USA).
ResultsManual analysisAll the evaluated parameters showed no significant differences between samples incubated at RT and 37°C (Table 1).
Seminal parameters obtained by manual analysis.
| Seminal parameters | Room temperature | 37°C |
|---|---|---|
| Total count (106) | 657.82±201.28 | 658.89±201.16 |
| Total motility (%) | 49.73±5.77 | 49.24±5.58 |
| Progressive motility (%) | 42.86±5.07 | 45.19±5 .48 |
| Sperm vitality (% alive) | 59.67±3.44 | 58.01±4.05 |
| Normal morphology (%) | 29.69±3.56 | 27.60±4.18 |
| Chromatin maturation (% mature) | 63.71±4.21 | 65.42±3.70 |
| Chromatin condensation (% condensed) | 85.83±2.55 | 81.68±2.82 |
| DNA fragmentation (% no fragmented) | 84.51±4.30 | 83.52±3.44 |
No statistical differences (P>0.05) were observed for any parameter.
All the kinematic parameters showed lower values when samples were incubated at RT (23°C), than at 37°C, independently of the temperature at which the analysis was carried out. When temperature of analysis was taken into account, at RT the movement was more linear (higher VSL, LIN and STR) and faster, particularly after incubation at 37°C (Table 2).
Effect of incubation and analysis temperatures on kinematic parameters of human spermatozoa.
| Kinematic parameters | Tincubation–Tanalysis | |||
|---|---|---|---|---|
| RT-RT | RT-37 | 37-RT | 37-37 | |
| N | 3233 | 2719 | 3767 | 3479 |
| VCL (μm/s) | 67.09±0.68a | 67.97±0.74a | 95.84±0.63b | 92.40±0.66c |
| VSL (μm/s) | 22.69±0.36a | 21.05±0.40b | 48.21±0.34c | 43.13±0.35d |
| VAP (μm/s) | 37.42±0.37a | 38.74±0.40a | 57.51±0.34b | 54.54±0.35c |
| LIN (%) | 35.56±0.33a | 32.21±0.36b | 50.04±0.30c | 46.25±0.32d |
| STR (%) | 60.31±0.41a | 53.51±0.45b | 79.19±0.38c | 74.30±0.40d |
| WOB (%) | 57.52±0.26a | 58.71±0.29b | 61.06±0.24c | 60.28±0.25c |
| ALH (μm) | 1.67±0.02a | 1.66±0.02a | 2.18±0.01b | 2.15±0.02b |
| BCF (Hz) | 12.62±0.11a | 11.82±0.12b | 16.82±0.10c | 15.32±0.10d |
Different letters in the same rows indicate significant differences (P<0.05).
It is remarkable that VSL, after incubation at 37°C and analysis at RT, exhibited twice the value of that after both incubation and analysis at RT. On the other hand, STR, the most common parameter used for defining the progressive movement of spermatozoa, was about 25% higher in the same comparison (Table 1). Thus, the differences were not only significant from the statistical point of view but also for the interpretation of results.
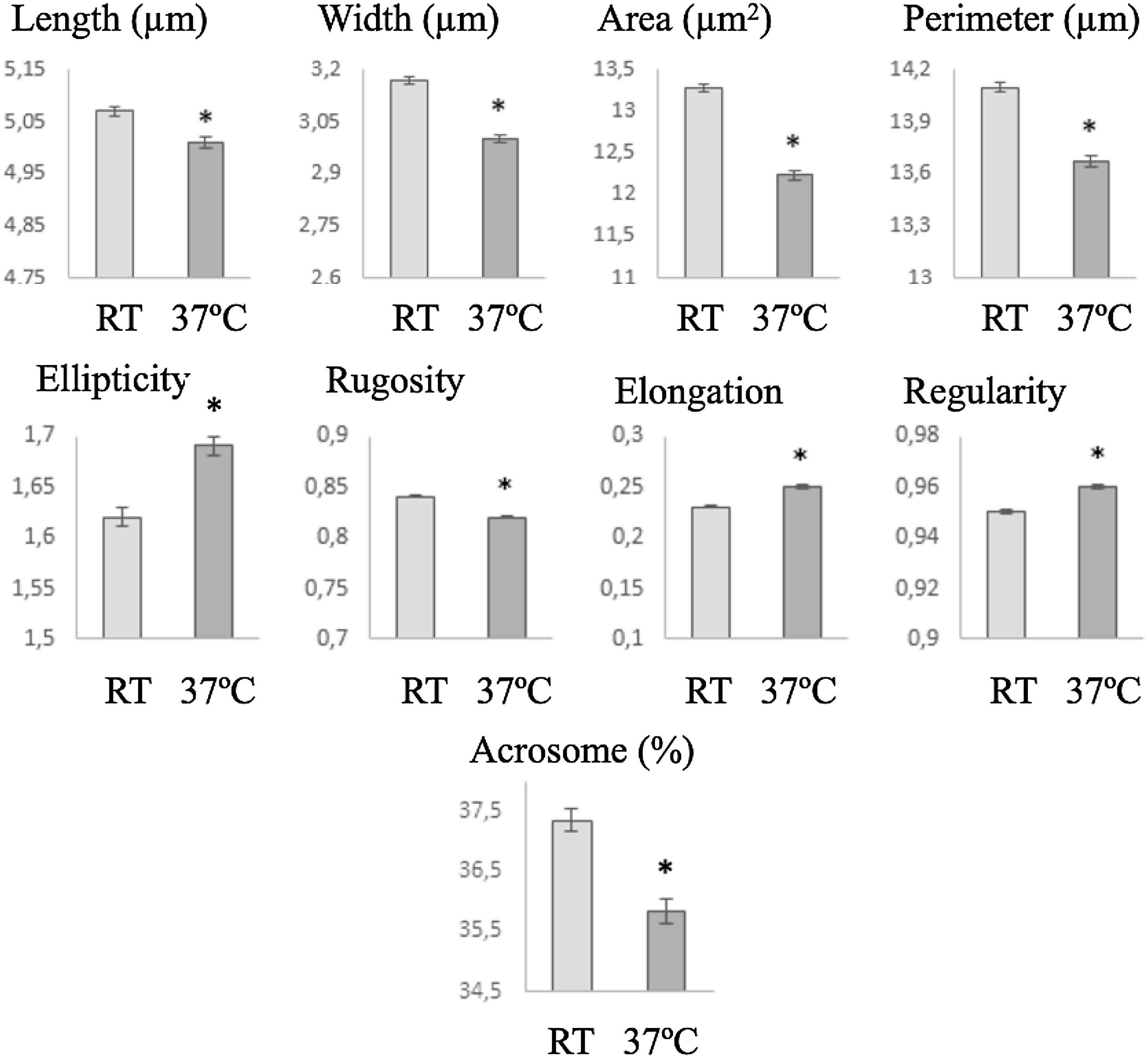
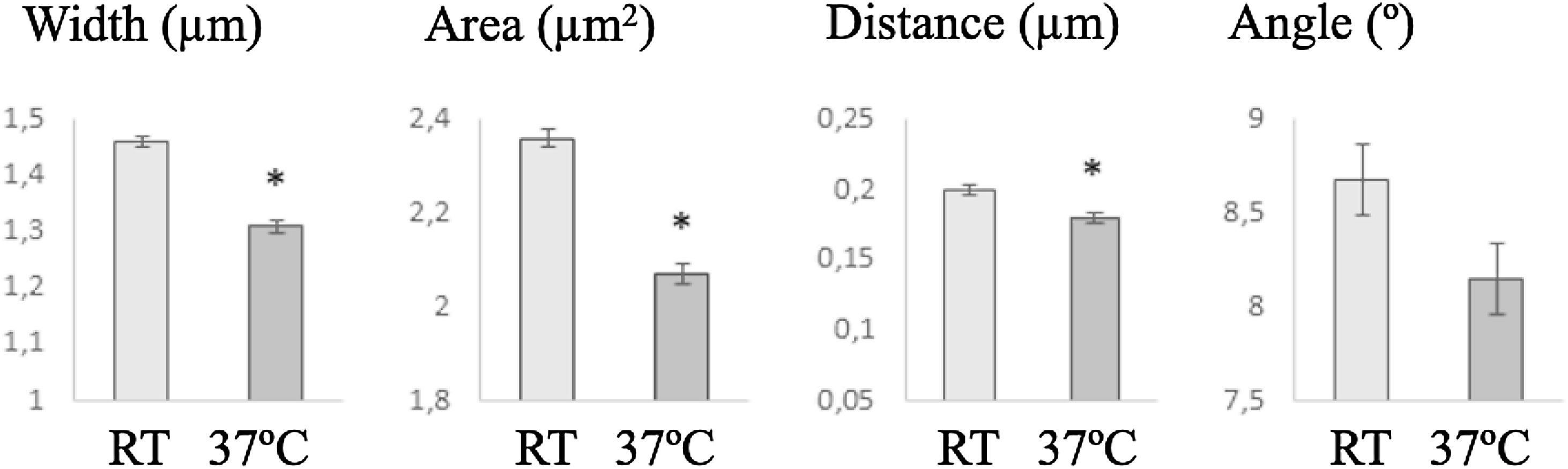
Morphometric analysisSperm head size was smaller after incubation at 37°C than at RT, but the ellipticity, elongation and regularity increased and heads become less regular (Fig. 4). In the same way, midpiece was bigger when the cells were incubated at RT, showing lower insertion distance and angle (in this case with non-significant differences) (Fig. 5).
The standardization of methods for semen analysis is a requisite to minimize technical variation allowing for a correct biological evaluation of the samples and to be able to compare among different samples of the same or different individuals, and between laboratories. The WHO manual39 only indicated that every laboratory must standardize procedures if they work at room temperature or at 37°C. The results presented here show that it is needed to define just one temperature to avoid bad interpretation of the results.
The way in which spermatozoa move is dependent upon cellular metabolism which is highly temperature dependent. While changes in temperature may not cause appreciable changes in the proportions of motile and progressive motile spermatozoa, which was observed also here, movement characteristics will be affected.25 At the beginning of sperm kinematics quantification era it was demonstrated how much velocity is sensitive to temperature changes, showing considerable increases when the sample temperature was raised from 25°C to 37°C.23
It was proposed that assessments one hour after liquefaction should use 37°C as the optimal temperature, compared with 4h and 25°C.9 When longer incubation times (from 3 to 18h) were considered it was recommended that semen must be kept at room temperature (20°C) and not in an incubator at 37°C.1 However, incubation for 18h at room temperature prevents human sperm capacitation.20 Longer incubation times (24h) revealed not only a motility damage effect of 37°C comparing with room temperature but an increase in sperm chromatin decondensation,13 DNA fragmentation index21,37 and sperm nuclear morphology, with an increase of vacuolated nucleus over time at this temperature.29 When, in addition to temperature effect, samples were subjected to density-gradient centrifugation and incubated for 24h, motility was higher when RT was used than 35°C.35 All these findings can be associated with the fact that lowering the temperature reduces the metabolic activity of spermatozoa, sustaining viability during long incubation times. This is also the reason for a reduction in temperature during transport of seminal doses in a variety of domestic animals.19 Therefore, several authors have recommended the use of room temperature (22°C) for sperm incubations during a period of two hours or more, when such incubation is needed in for ARTs in human samples.29
Comparative studies in other mammalian species, showed better results for motility and basic kinematic parameters after incubation at 20°C than at 37°C in goat samples,12 although the most common temperature during analysis in this species is 37°C.18 In the case of dog, the temperature of 38°C rendered better motility and kinematics results than 30°C.14 With respect to morphological changes, in goat samples the incubation for 3min at 20°C showed lower number of head morphological abnormalities than after incubation at 37°C; in any case, nevertheless these differences were not significant.12
The results presented here, in accordance with previous observations,23 showed higher velocities and linear motility after incubation at 37°C than at RT but, interestingly, values were higher when the analysis was carried out at RT. The question that arises is what is closer to the real motility of the cell in the natural conditions, that is, inside the vagina and the rest of the female reproductive tract. There is a general tendency to assume that higher values indicate better analysis conditions, but this may not be true. In fact, these values could relate to an artefactual increase of cellular metabolism rather than to optimal conditions in the analysis. What is clear is that the differences observed here indicated that it is necessary to standardize the analysis technique and conditions, and taking into account that spermatozoa evolved to perform at the temperature of the female genital tract, which in humans is closer to 37°C than the alternative RT.
Regarding the effect of temperature of incubation on sperm morphology the literature is very scarce. Sperm nuclear vacuolization was unaffected by incubation temperature (RT and 37°C for 4h) in motile spermatozoa after preparation and isolation by swim-up.31 Incubation of samples at RT for 24h rendered a higher proportion of spermatozoa with normal morphology than incubation at 35°C.35
Staining procedure introduces an artefactual cellular process consequence of the dehydration after smearing and posterior use of different chemicals to stain the spermatozoa.4,5,40,34 So, the differences in morphometry observed here after incubation at RT and 37°C could related with the interaction of spermatozoa with the staining procedure but not necessarily with a prior in vivo change in the size of the cells. In any case, the only changed variable in this experiment was the temperature and thus that potential artefacts production was related with temperature effect. On the other hand, when overall subjective morphology was assessed no changes were observed. This is not surprising because subtle, even significant, changes in size cannot be appreciated by human visual perception and no comparisons among different slides can be made during analysis. Nevertheless, the significant changes observed here in the morphometry indicate a significant lower value for the size parameters, in both head (including a reduced acrosome) and midpiece, and an increment in the elongation of the sperm heads after incubation at 37°C. These differences can relate to changes in the composition or the fluidity of the plasma membrane which may vary according to incubation temperature. These observations reinforce the role of the CASA-Morph technology in the evaluation of changes in sperm morphology that cannot be appreciated subjectively, but that have a major significance for an adequate evaluation of sperm function.33
The integration of CASA technology into the world of clinical diagnostics has been limited, and in many cases nonexistent.11,36 One of the reasons for this fact (as opposed to the general automation of analyses which is most common in clinical laboratories around the world) relates to the fact that CASA results are not very different from human subjective analysis when CASA technology is used just for substituting manual analyses. It is generally held that estimating percentages of motility or normal morphology following human criteria, but with the aid of computational technology, does not justify the price of a CASA system.26 In contrast, when CASA technology is used for quantitative analysis of sperm characteristics, and all the technological limitations are adequately considered,3 the results to be obtained offer a clear advantage to advance towards better clinical diagnostics for male fertility.
ConclusionsThe subjective analysis of samples did not reveal differences after incubation at RT and 37°C. On the other hand, results of both kinematic and morphometric CASA assessments showed clear significant differences. We can conclude that CASA technology is needed to adequately evaluate subtle changes in sperm behaviour regarding temperature changes and recommend that both incubation and analysis is done at 37°C, the physiological temperature in the female tract. In any case, it is necessary to standardize the temperature used across laboratories to avoid different results when CASA technology is used. Otherwise, even using the same set-up for defining progressive motility can result in completely different diagnosis of the same sample.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were carried out on humans or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Authors’ contributionsConceptualization, AGM, NG and CS; methodology and investigation, AGM, CC and NN; validation, AGM and SS; Formal and statistical analysis, AV; writing, AGM, ERSR and CS. All authors have red and agreed to the published version of the manuscript.
Conflicts of interestThe authors declare no conflict of interest.