Androgens have been used to treat bone marrow failure syndromes such as aplastic anaemia (AA) since the 1970s, and are currently used as adjuvants to other treatment regimens.
ObjectiveTo determine the efficacy and response time of androgens in the treatment of AA.
Materials and methodsA retrospective, analytical-observational nested study in a cohort of patients with AA. The study was conducted from January 2006 to December 2013.
ResultsA total of 63 patients with a mean age of 47 (18–83) years were included in the study. Out of 27 patients in full remission, 17 received some type of immunosuppressant combined with androgens. The use of more than 2 immunosuppressants did not significantly improve response times (p=0.311, 95% CI). Mean response time was 725 (331–1119) days; 4-year survival rate was 86%.
ConclusionsImproved immunosuppressant response rates with adjuvant androgens were mainly observed in patients with severe AA.
Desde los sesenta se utilizan andrógenos para tratar síndromes de falla medular, como la anemia aplásica (AA), actualmente son complemento a otros tratamientos.
ObjetivoEstablecer la eficacia y tiempo de respuesta al tratamiento con andrógenos en AA.
Material y MétodosEstudio retrospectivo, observacional-analítico anidado en la cohorte de pacientes portadores de AA durante enero 2006 a diciembre 2013.
ResultadosSe estudiaron 63 pacientes, con edad media de 47 (18–83) años. De 27 pacientes (42.9%) que integraron remisión completa, 17 recibían algún inmunosupresor en conjunto con andrógenos. Usar más de 2 inmunosupresores elevó no significativamente (p=0.311, 95%IC) las respuestas. La media de respuesta fue de 725 (331–1,119) días. La supervivencia a 4 años fue del 86%.
ConclusionesAdicionar andrógenos mejoró la tasa de respuesta a los inmunosupresores principalmente en pacientes con AA muy severa.
Aplastic anaemia (AA) is an immune disease characterized by varying degrees of peripheral cytopaenia and fatty replacement of haematopoietic tissue.1 Response to immunosuppressants varies, but cyclosporine plus anti-thymocyte globulin (ATG) generally achieves a response rate of over 60%.2,3 The combination of haematopoietic stem cell transplant and immunosuppressant therapy has increased the survival rate of AA patient to over 80% worldwide, with a second cycle of ATGs or administration of new thrombomimetic drugs being reserved for refractory cases.4,5 Prior to these therapeutic advances, evidence from a number of case series led to androgens being included in regimens to treat bone marrow failure syndromes.6,7 In the 1970s, attempts were made to combine them with other options such as steroids,8,9 but it was not until the end of that decade that they were first combined with haematopoietic stem cell transplant.9,10 In the mid 1980s, Champlin et al. published the first prospective study in 121 patients receiving androgens as adjuvant to ATGs. The study found no significant differences in remission rates.11 Following this, interest in androgens faded, and their use was limited to a few small series. In Mexico, Pérez et al. evaluated the efficacy of danazol as first line therapy, and reported a mean response time of 3 months, with an overall response rate of 46%.12 In the General Hospital of Mexico, standard treatment is based on immunosuppressants in combination with different types of androgens. The main aim of this study has been to evaluate combination therapy with androgens and immunosuppressants in terms of response time and rate.
MethodsStudy designWe carried out a retrospective chart review of patients with acquired AA seen in the General Hospital of Mexico from January 2006 to December 2013.
All patients meeting diagnostic criteria for AA whose primary treatment had included androgens alone or in combination with immunosuppressants. Diagnosis of AA was defined by at least 2 of the following criteria: haemoglobin less than 10g/dL, platelet count less than 50×109/L, neutrophil count less than 1.5×109/L and hypercellular bone marrow.
Exclusion criteria were: incomplete medical record, therapy including bone marrow stimulants, evidence of paroxysmal nocturnal haemoglobinuria (PNH) with haemolysis, history of thrombosis associated with PNH or clone greater that 50% on flow cytometry, pregnancy, or prior diagnosis of malignancy.
The clinical and laboratory reports of study patients at the time of diagnosis, together with the therapy used, the clinical course, transfusion requirements, and date of the last visit were recorded. Cases were categorized as moderate and severe AA according to severity, which was assessed on the basis of neutrophil and platelet count at the time of diagnosis. The severe group was later divided into severe and very severe.13
Treatment protocolThe immunosuppressant cyclosporin A (CpA) was administered daily (5mg/kg) to maintain levels of 150–250ng/mL. Other immunosuppressants used in combination with CpA were mycophenolate mofetil and prednisone (1mg/kg). Red blood cell (RBC) or platelet transfusion was ordered at the discretion of the attending physician. Response to treatment was evaluated on the basis of established criteria.13
AndrogensThe androgens most commonly used were danazol and mesterolone, followed by testosterone and oxymetholone. Danazol was given orally at 200mg every 8h, and was either stepped down or suspended at the discretion of the attending physician.
Ethical considerationsBeing a retrospective study, the data were sourced from medical records held in the Haematology Departments. Access to these was strictly controlled, in accordance with regulations in force in Mexico, and patient privacy and anonymity was maintained at all times.
Statistical analysisStudy data were analyzed using IBM SPSS version 20.0 (Armonk, New York). Overall survival was estimated using Kaplan–Meier curves; response of each severity category was estimated using the log rank test. Inter-group comparisons were estimated using the chi-squared test; significance was set at p≤0.05 (5%).
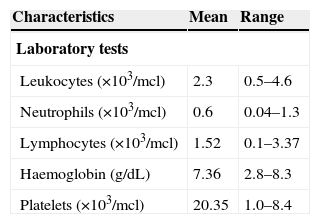
ResultsOf the 63 patients included in the study, 3 were excluded due to a large PNH clone size. The mean age was 47.7 years (range 18–83 years); 52.4% of patients were men (n=33) and 47.6% were women (n=30). Table 1 shows the characteristics of the patients at the time of diagnosis.
General characteristics of patients.
| Characteristics | Mean | Range |
|---|---|---|
| Laboratory tests | ||
| Leukocytes (×103/mcl) | 2.3 | 0.5–4.6 |
| Neutrophils (×103/mcl) | 0.6 | 0.04–1.3 |
| Lymphocytes (×103/mcl) | 1.52 | 0.1–3.37 |
| Haemoglobin (g/dL) | 7.36 | 2.8–8.3 |
| Platelets (×103/mcl) | 20.35 | 1.0–8.4 |
| Characteristics | (n=) | (%) |
|---|---|---|
| Risk group | ||
| Moderate AA | 23 | 36.5 |
| Severe AA | 16 | 25.4 |
| Very severe AA | 24 | 38.1 |
| Karyotype | ||
| Not available | 24 | 38.1 |
| Normal karyotype | 36 | 57.2 |
| 46 XX del 7q | 1 | 1.6 |
| 46XX polyploid | 1 | 1.6 |
| Trisomy 19,16 and 18 | 1 | 1.6 |
| PNH clone<50% | 4 | 6.3 |
Abbreviations: AA: aplastic anaemia; PNH: paroxysmal nocturnal haemoglobinuria.
The most commonly used immunosuppressant was CpA (87%), both alone or in combination with other immunosuppressants. Prednisone was given at some time to 13% of patients, and 17% received mycophenolate mofetil. Only 3% received rabbit anti-thymocyte globulin. As the regimen has not been standardized, patients were given various types of androgens, with danazol being the most commonly used (95%). Nearly 75% of patients given oxymetholone as primary therapy had switched to danazol by the end of their treatment. In terms of primary therapy, 73% (n=46) were given danazol, 20.6% (n=20.6) mesterolone, 4.8% (n=3) oxymetholone, and 1.6% (n=1) testosterone.
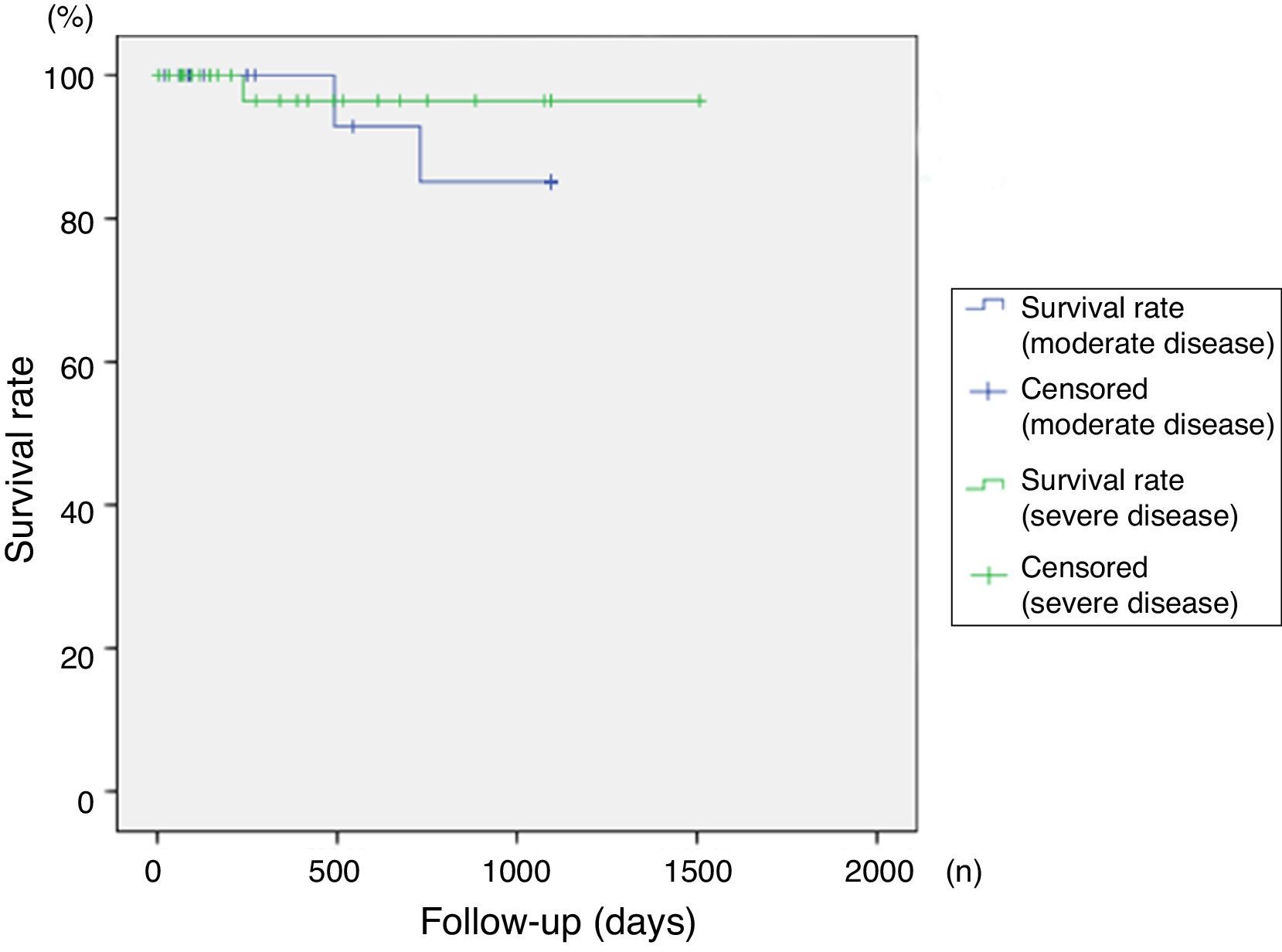
Follow-up and responseMean follow-up was 683 days (150–1507 days). Mean response time was 745 days in the severe group (severe/very severe) and 341 days in the moderate group. In the severe group, 45% (n=18) of patients classified as severe (as opposed to very severe), responded to treatment, vs. 39.1% in the moderate group. Although response varied between groups, the differences were not statistically significant (p=0.427, 95% CI). In total, 50.8% of patients took a combination of 2 or more immunosuppressants, mainly mycophenolate and cyclosporine, while 49.2% received monotherapy, mainly prednisone or cyclosporine A. No significant differences in type of response were observed between monotherapy and combination therapy (p=0.182, 95% CI).
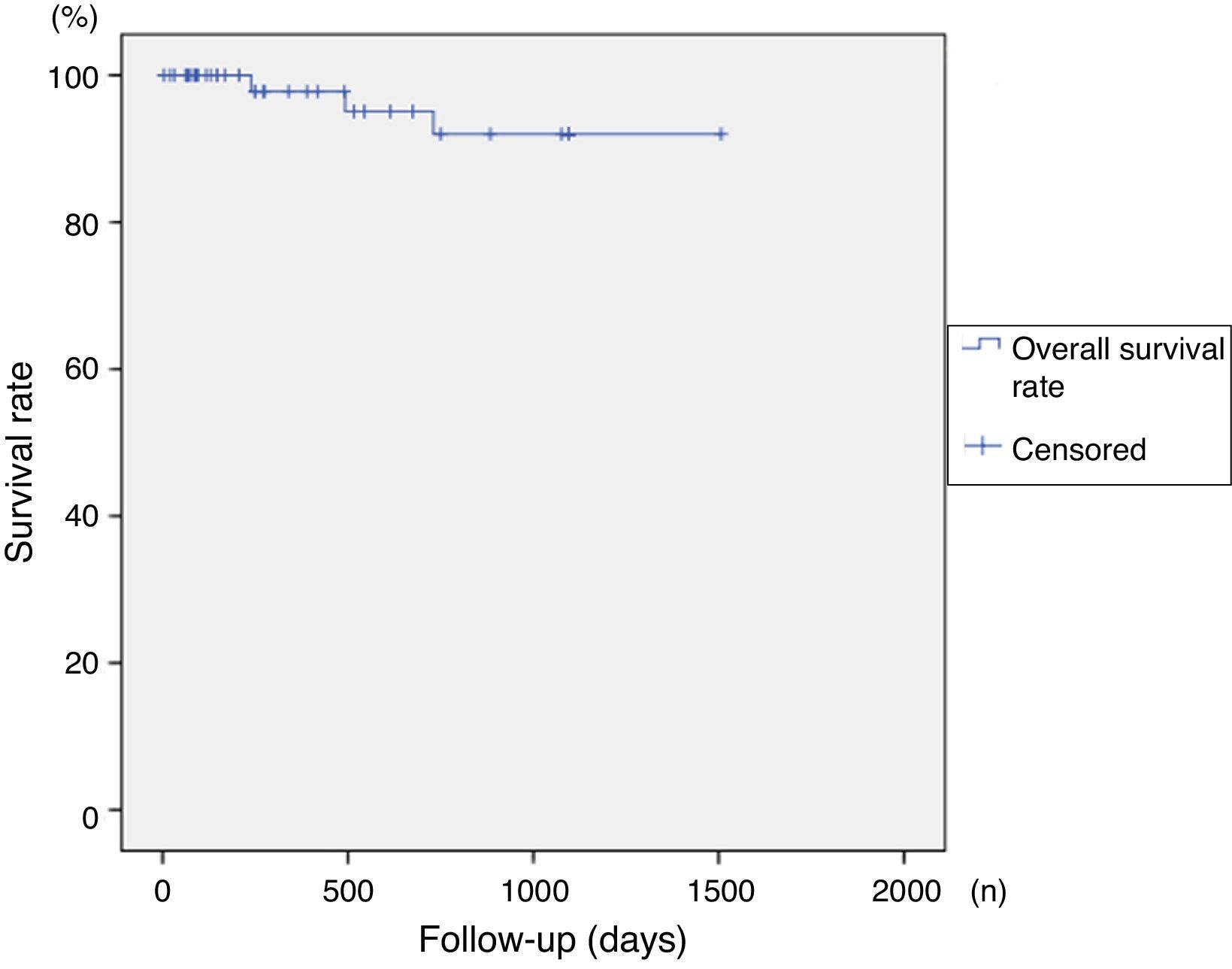
SurvivalOverall survival rates exceeded 86% at 4 years of follow up, with no significant differences between groups in terms of severity of disease (p=0.315). Survival curves are shown in Figs. 1 and 2.
TransfusionsPatients in the moderate group received a mean of 11.04 RCB transfusions vs. 11.89 in the severe group. The most disperse data were found in the severe group (variance: 53.04 vs. 73.49; interquartile range: 5 vs. 9.75), and for this reason the group was divided into severe and very severe cases. Having divided the group, we were able to observe that although severe AA patients required more transfusions, even exceeding very severe patients (14.37 vs. 10.28), most of these were probably due to atypical cases (interquartile range: 10.5 vs. 11.75) (see Table 2).
Transfusion requirements during treatment for aplastic anaemia.
| Moderate AA | Severe AA | |
|---|---|---|
| Number of bags | 11.04 (7.8–30) | 11.89 (9.1–73) |
| Interquartile range | 5 | 9.75 |
| Variance | 53.04 | 73.49 |
| Moderate AA | Severe AA | Very severe AA | |
|---|---|---|---|
| Number of bags | 11.04 (7.8–30) | 14.37 (9.7–34) | 10.28 (9.1–73) |
| Interquartile range | 5 | 10.5 | 11.75 |
| Variance | 53.04 | 75 | 68.43 |
The mechanism of action of androgens in bone marrow failure syndromes remained obscure for over 30 years. The studies published by Sánchez-Medal in the mid-1970s described how different types of androgens could improve cytopaenia in aplastic anaemia, mainly in patients with moderate disease.14,15 Interest in the therapy gradually faded, until it was eventually ignored in most therapeutic guidelines.13,16 Although evidence in the literature has consistently shown the scant beneficial therapeutic effect of androgens, most physicians continue to use it regularly, either in monotherapy or in combination with an immunosuppressant. Our hospital has gradually introduced a range of therapeutic strategies for management of aplastic anaemia (prednisone, cyclosporine, anti-CD52, anti-thymocyte globulin). Androgens are always included in these strategies, and are given in both the active stage of the disease and following remission. We were unable to establish an association between androgen administration and response to immunosuppressive therapy. The only difference we observed was related to response time, which was found to be much faster in patients with moderate vs. severe disease. Unfortunately, due to the lack of randomized clinical trials, androgens are now largely ignored, as the findings of existing studies have shown that they are most useful in patients with blood disorders (18). In contrast to the clinical evidence, however, significant advances have been made in the past 10 years. The lack of clinical evidence led to androgens being largely side-lined, particularly in recent years. However, new research in the early 2010s identified a new mechanism of action that explained the activity of androgens in marrow failure syndromes. Young et al. identified the role played by telomere shortening in bone marrow failure syndromes17. The function of these structures located at the end of linear chromosomes is to protect the chromosome and prevent loss of genetic material. However, when they are shortened, the cells can become prone to apoptosis, senescence, and in some cases, malignant transformation.18 The best example of a telomeropathy, or disease caused by telomere shortening, is dyskeratosis congenita19, in which all telomere-binding proteins (TERT, TERC, DKC1, NOP10, TINF2) are altered.20 Calado et al. confirmed the effect of androgens on telomerase expression in in vitro studies, finding that exposure to androgens restored the telomerase expression and activity of cells from patients with mutations affecting telomerase activity.21 Yamaguchi et al., meanwhile, suggested that telomerase shortening or mutations may be caused by haploinsufficiency.22 Other mechanisms have mainly been associated with erythropoietin-mediated haemoglobin synthesis. Sex hormones are known to affect renal microvasculature, insofar as oestrogens dilate and androgens constrict blood vessels, and this action affects both venous and capillary haematocrit levels. These changes could explain haemoglobin differences between men and women.23,24
Our study is limited, as the data were collected from clinical records after implementation of the particular therapeutic strategy, and we had no control over the choice of immunosuppressants. Randomized, preferably case-control clinical trials are needed to corroborate our findings.
In conclusion, although our findings do not show that androgens affect response in patients with aplastic anaemia, new clinical evidence has shown the therapeutic potential of these hormones. Randomized studies are needed to definitively establish the efficacy of androgens in both monotherapy and in combination with other immunosuppressive regimens.
FundingThis study was funded by the General “Dr. Eduardo Liceaga” Hospital of Mexico, without the help of external sponsors or pharmaceutical organizations.
Conflict of interestThe authors declare they have no conflict of interest.