Brain metastases frequently occur secondary to poorly controlled primary lung, breast, melanoma, colorectal and renal tumours. Because of this, correct diagnosis and early treatment of brain metastasis is difficult, which is why the condition should be treated and controlled by a multidisciplinary medical team so as to obtain real-life and reliable statistics and to lower the incidence of brain metastasis, improving the quality of life of patients and reducing mortality rates.
La enfermedad metastásica cerebral (EMC) es una patología, frecuente sobre todo en tumores primarios de pulmón, mama, melanoma, colorrectal y renal descontrolados. El diagnóstico oportuno y tratamiento temprano, es la falla sobre la EMC, debido a la misma causa. Por lo que debería ser manejado con un equipo médico multidisciplinario, para poder controlar, obtener estadísticas reales, y fidedignas, y así bajar la frecuencia de EMC, mejorando la calidad de vida de los pacientes, y reduciendo las estadísticas de mortalidad.
The real incidence of metastatic brain tumours remains unknown,1,2 although some countries such as the United States of America report 17,000 cases annually,1 and some authors consider brain metastases such as intracranial tumour to be more common.
Brain metastases occur in 15–40% of patients with systemic cancer, which represents approximately 12 patients per 100,000 inhabitants per year.3–5
It is usually diagnosed between the 5th and 7th decade of life. This concurs with statistics from the American Cancer Society, which reveal that the probability of developing invasive cancer is high between 60 and 79 years of age.6
Even after performing a thorough study to identify the primary tumour, it remains undetected in 15% of patients.7
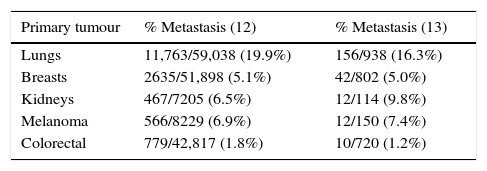
Certain types of cancers are more prone to spread to the central nervous system.8 The most common, in order of frequency, are lung cancer, breast cancer, melanoma, colorectal and renal cancer, and less frequently thyroid cancer, gastrointestinal cancer and prostate cancer. Haematological neoplasms constitute only 10% of brain metastases and primarily affect the leptomeninges9–11 (see Table 1).
Incidence of brain metastasis.
| Primary tumour | % Metastasis (12) | % Metastasis (13) |
|---|---|---|
| Lungs | 11,763/59,038 (19.9%) | 156/938 (16.3%) |
| Breasts | 2635/51,898 (5.1%) | 42/802 (5.0%) |
| Kidneys | 467/7205 (6.5%) | 12/114 (9.8%) |
| Melanoma | 566/8229 (6.9%) | 12/150 (7.4%) |
| Colorectal | 779/42,817 (1.8%) | 10/720 (1.2%) |
Refs. 10, 11.
Most patients’ initial manifestations are neurological, and therefore their first point of contact is commonly with a neurosurgeon or neurologist before they are referred to an oncologist.12 It is considered that 15% of patients in whom the primary tumour has been identified at the time of diagnosis and 45% of these who moreover present neurological symptoms will be diagnosed with metastatic brain tumour.13,31
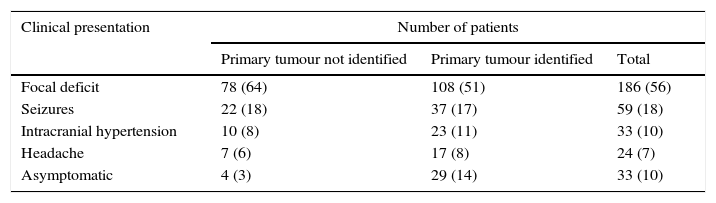
The most common symptoms include (see Table 2) headache, weakness, alterations of higher brain functions, focal neurological deficit and seizures, which are caused due to a local mass, cerebral oedema and increased intracranial pressure.14
Clinical manifestations in patients with brain metastasis (Neurosurg. Focus/Volume 22/March, 2007).
| Clinical presentation | Number of patients | ||
|---|---|---|---|
| Primary tumour not identified | Primary tumour identified | Total | |
| Focal deficit | 78 (64) | 108 (51) | 186 (56) |
| Seizures | 22 (18) | 37 (17) | 59 (18) |
| Intracranial hypertension | 10 (8) | 23 (11) | 33 (10) |
| Headache | 7 (6) | 17 (8) | 24 (7) |
| Asymptomatic | 4 (3) | 29 (14) | 33 (10) |
Values in brackets represent percentage of symptoms.
Nuclear magnetic resonance imaging (MRI) of the brain is considered to be the exam of choice in patients with clinical suspicion of brain metastasis because it has a high sensitivity and specificity.15
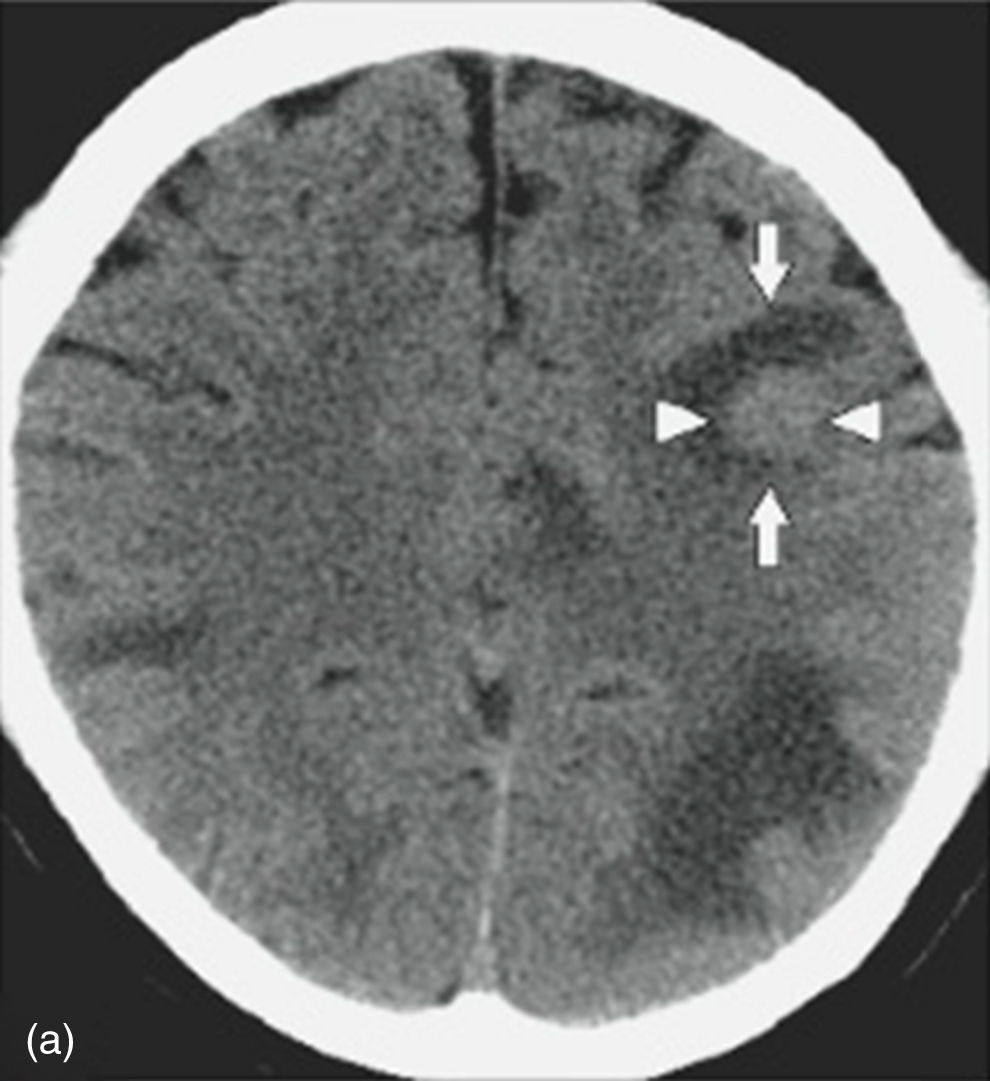
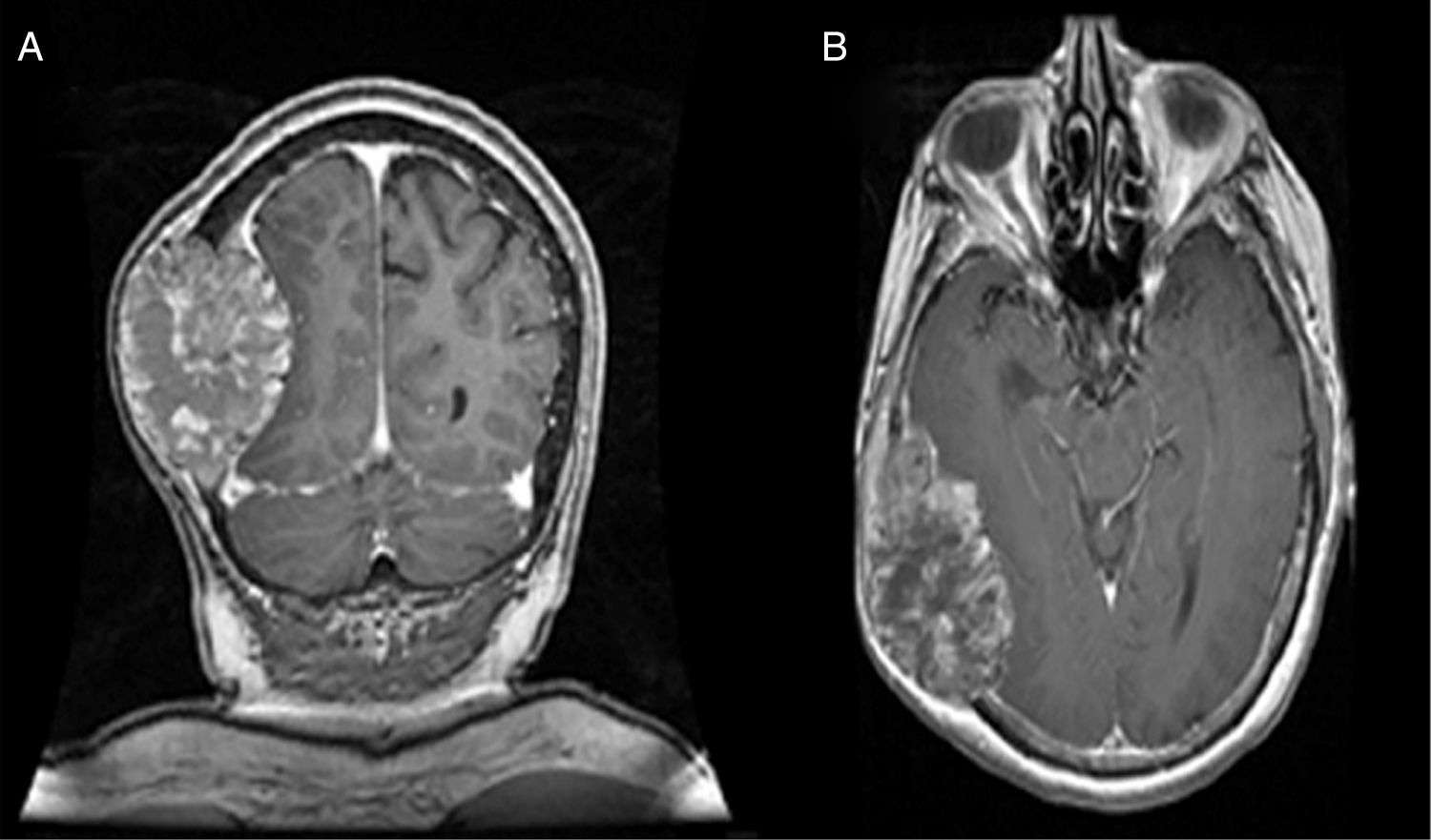
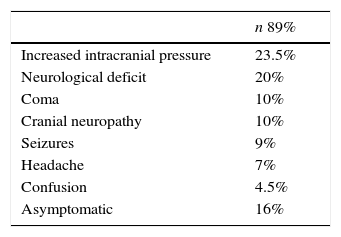
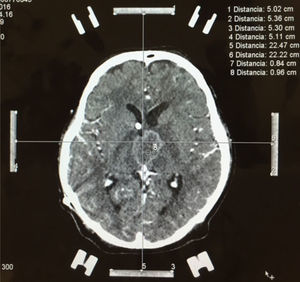
Nevertheless, in patients with a contraindication for MRI, computed axial tomography (CAT) scan of the brain or PET (positron emission tomography) are the favoured options. CAT scan of the brain can be performed with or without a contrast medium to reveal metastatic lesions that are isodense to the brain parenchyma (Fig. 1), whilst a hyperdense image suggests the presence of haemorrhage, which could lead to the diagnosis of lung tumour, renal tumour, thyroid tumour, choriocarcinoma or melanoma metastasis. Moreover, it determines whether the patient has hydrocephalus, brain oedema or brain herniation. When a contrast medium is administered the lesion is enhanced and multiple lesions become visible.16,17,26
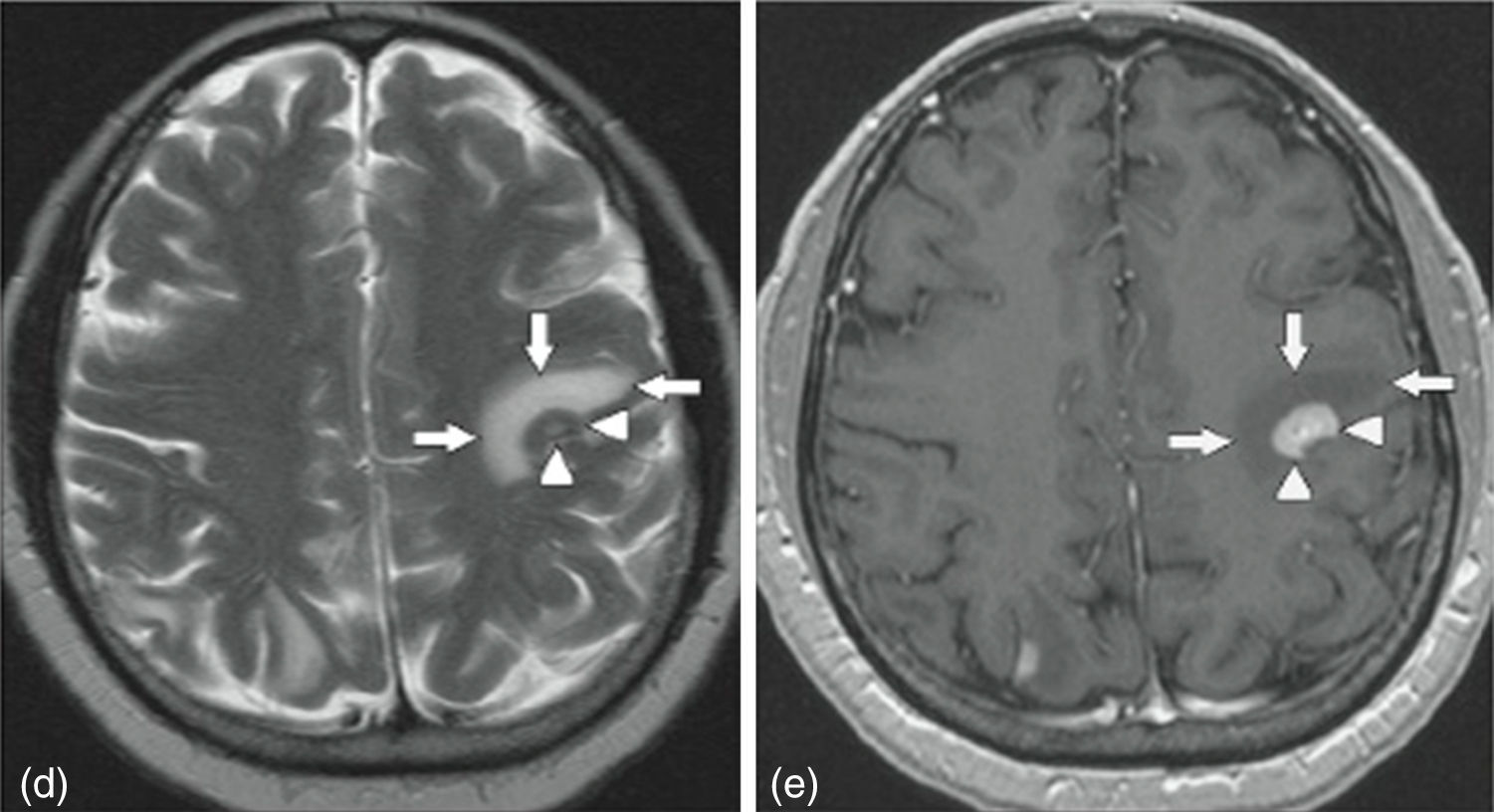
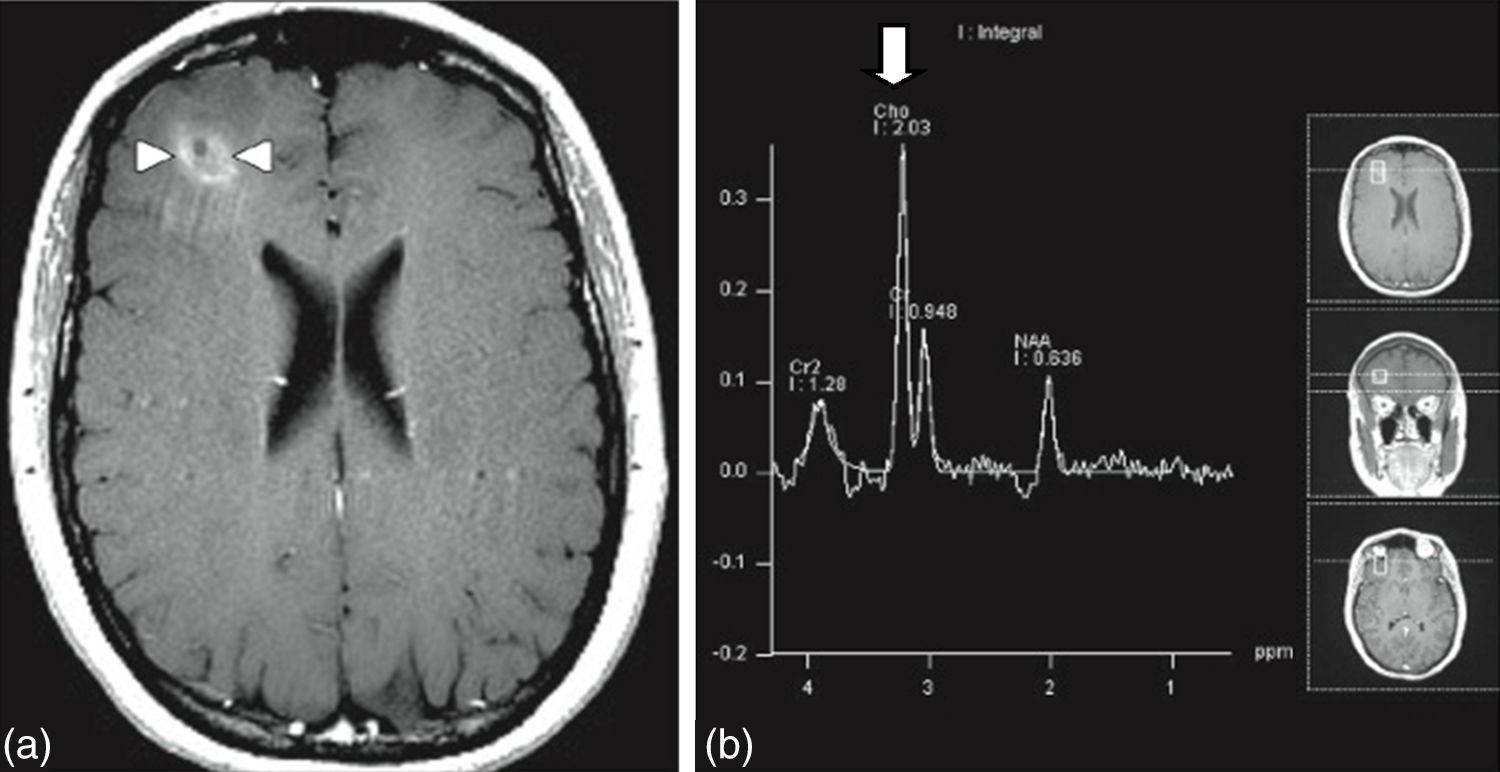
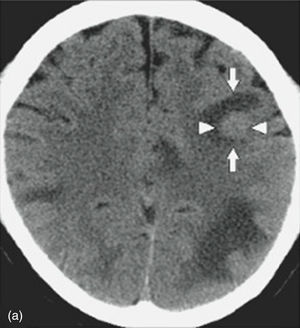
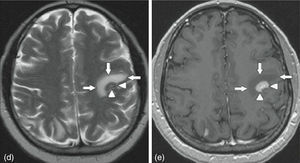
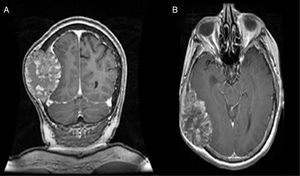
MRI of the brain employs basic T1- and T2-weighted sequences, FLAIR, diffusion and perfusion, in addition to spectroscopy and tractography. Most metastatic lesions are hypointense on T1-weighted images, which could be indicative of haemorrhage, melanin and necrosis (Fig. 2). On T2-weighted images most lesions are hyperintense. Bleeding appears as hypointense when in acute state, and hyperintense when subacute, while vasogenic oedema is hyperintense.18 Spectroscopy is a magnetic resonance imaging technique that enables the metabolic characteristics of the lesion to be evaluated. It can be represented in 2D (two dimensional) or 3D (three dimensional) (Fig. 3), detecting the ranges of spectra of normal brain tissue, metastasis, necrosis, gliosis and vasogenic oedema.19,20 PET and SPECT (single-photon emission computed tomography) used for the diagnosis of brain metastasis are limited, due to their low sensitivity for detecting small lesions or potentially recurring tumours, and PET is limited to differentiating between necrosis due to radiation or recurrence.21,22 Complementary studies such as chest–abdomen–pelvis CT scan, chest X-ray, bronchoscopy, CARBOWAX and tumour markers are conducted according to the clinical suspicion of the disease.
The use of brain resonance is recommended as part of the evaluation for some patients with tumours that have already been diagnosed, those on treatment and those in follow-up.22
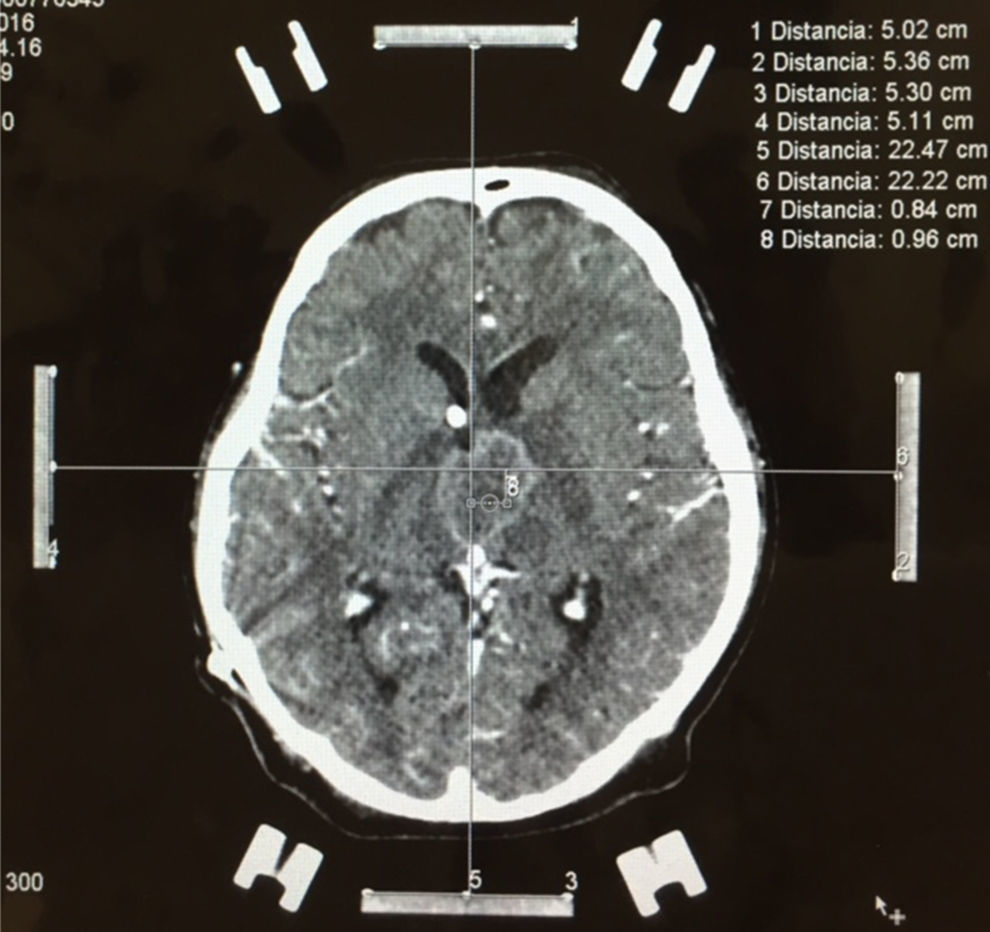
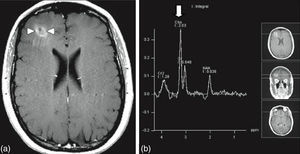
Stereotactic biopsy for the diagnosis of metastatic brain tumoursFor multiple lesions located deep in the brain or in highly specialised regions (language area, primary motor area, visual area) and when the primary tumour has not been identified in other studies, stereotactic biopsy is a valuable diagnostic resource, with a mortality rate of less than 1% (Fig. 4).
RPA (recursive partitioning analysis) classificationThe RTOG (Radiation Therapy Oncology Group) developed a statistical method to categorise cancer patients, called the RPA classification system. It contemplates several factors such as patient age, Karnofsky Performance Status (KPS) and control of primary tumour, to better understand the prognostic factors for selecting patients who will benefit from surgery. Patients with RPA class 1 are under 65 years of age, with a KPS greater than 70% and control or absence of systemic disease. RPA class 2 patients are older than 65 years of age with a KPS greater than 70% and uncontrolled systemic disease. RPA class 3 patients have a KPS under 70%.
RPA class 1 patients are considered to be good candidates for craniotomy and resection, while RPA class 3 patients are the worst candidates for surgical treatment. The selection of RPA class 2 patients is less clear, requiring survival time and medical risk factors for surgery to be taken into account.23
TreatmentTreatment consists of surgery, radiotherapy, radiosurgery, chemotherapy and drugs such as antiepileptics and corticosteroids. Antiepileptic drugs, such as phenytoin (DPH) at a starting dose of 15–17mg/kg and a sustained dose of 5–7mg/kg, have been shown to be prophylactic treatment for generalised seizures, and other anticonvulsants have been used for focal seizures, such as carbamazepine or oxcarbazepine.24 The corticosteroid of choice in the management of vasogenic cerebral oedema is dexamethasone, recommended for moderate symptoms at a dose of 4–8mg/day. For severe symptoms the dose may be increased up to 16mg/day, with a duration of treatment no longer than 2 weeks.25
SurgeryIt provides different advantages over other treatments: firstly, complete resection immediately removes the mass effect, brain irritation and vasogenic cerebral oedema; secondly, surgery provides tissue to be sent for histopathological study in case the primary tumour is unknown; and thirdly, complete surgical resection provides local cure.27,28 Occasionally the risks of surgery exceed the benefits, such as: secondary neurological deficit due to lesion in an eloquent area, meningitis, brain abscess, intracranial haemorrhage or even death. Nevertheless, thanks to modern techniques and sophisticated technology, surgical mortality for brain metastases has dramatically decreased since the initial reports of craniotomies, and is now below 3%. The risk of haemorrhage or neurological deterioration associated with surgery is less than 5%, while the risk of meningitis and brain abscess is less than 1%. Non-surgery-related complications, such as infection, deep vein thrombosis, pulmonary embolism and pneumonia, occur in 8–10% of patients.28–30
Treatment protocols have been defined for single and multiple brain metastases, from which the following can be concluded: Patients with systemic disease and single brain metastasis should be treated with surgical resection plus whole brain radiotherapy. This has led to an increase in survival, fewer recurrences and greater quality of life.33 Patients with three lesions or fewer, with good systemic control, should also be treated with surgery and whole brain radiotherapy, as the outcome is the same as for patients treated for single metastasis.32 Despite advances in surgery and the safety of the anaesthesia technique, patients with more than three lesions have a particularly poor prognosis and are not good candidates for surgery. Data obtained from several retrospective studies show that for patients with controlled systemic disease and more than three surgically accessible lesions, surgical intervention should be discussed with a good neuro-oncology team.33
The management of recurring metastases should be as per the most recent references similar to the newly diagnosed brain metastasis, as it is considered to have the same outcome.34
RadiotherapyThe optimal dose for managing patients with brain metastasis includes 10 sessions of 3Gy (30Gy) split over two weeks or 15 sessions of 2.5Gy (37.5Gy) split over three weeks. These variations in dose and time have not demonstrated superior results; in fact, dose and time increases have been shown to cause more adverse effects.
Complications caused by radiotherapy are classified as acute (under 90 days) and chronic (more than 90 days). Acute complications include hair loss, dermatitis, otitis externa, hearing loss, nausea, vomiting and drowsiness. Chronic complications include tissue necrosis, personality changes, memory loss, cerebellar disorders, cataracts and deterioration in cognitive function.35
RadiosurgeryThe main candidates for this treatment are those patients who do not meet the criteria for surgical treatment, those with lesions under 3cm or lesions located in eloquent areas, and those with residual lesion after resection. The treatment parameters imply that the maximum tolerated dose depends on the diameter of the lesion: 15Gy for tumours under 20mm and 20Gy for tumours 31–40mm.36,37
ChemotherapyThe role of chemotherapy in the treatment of patients with brain metastasis is not clearly defined. Currently, chemotherapy is used very little in the management of patients not included in research protocols, with the exception of very chemo-sensitive tumours, such as small cell lung carcinoma, germ cell tumours and lymphoid neoplasms.38
PrognosisThe prognosis of brain metastasis patients in the last two decades with the use of combined therapies shows an increase in survival compared to patients who only received radiotherapy or surgery. The final result of the studies conducted shows that surgery plus radiotherapy presents an increase in survival of 7–9 months.25,29,39
Dural metastasesThese usually affect patients at advanced disease stages and the mechanisms of dissemination are direct extension or haematogenous spread.
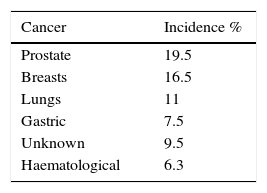
The incidence of dural metastases has increased in recent decades as a result of developments in new neuroimaging techniques and an increase in the survival of cancer patients due to improved therapies. Most patients are asymptomatic; however, they may debut with progressive neurological deficit.33,34 The most recent study in the literature review was published by Laigle-Donadey, covering 198 cases from 1994 to 2003. The patient age range was from 4 months to 84 years (mean of 59 years) and the most common dural metastases reported (in order of frequency) were prostate, breast, lung and stomach cancer, making up 54.5% (108 patients). The exact incidence of dural metastases is difficult to ascertain39 (see Table 3).
Clinical manifestationsThe symptoms presented are usually related to brain compression or invasion, production of subdural haematomas or fluid collection, and less frequently due to occlusion of branches to the dural sinuses. Approximately 20% of patients are asymptomatic, and the lesions are identified in a follow-up study or in necropsy38 (see Table 4).
Non-traumatic subdural haematomas resulting from neoplastic invasion of the meninges are a classic complication, with an incidence observed in 15–40% of cases.37,38
DiagnosisThe exam of choice is contrast-enhanced MRI of the brain (Fig. 5). The classic image of dural metastasis is a mass with homogeneous enhancement on T2-weighted images, originating in the dura mater. When the dural tail sign appears, a differential diagnosis should be made for meningioma, particularly in patients with prostate cancer, which also causes a hyperostotic effect.36
TreatmentSurgical resection is normally performed when there is a single, well defined lesion that is surgically accessible, with adequate control of extracranial disease. Surgical resection is recommended in patients with progressive systemic disease but good quality of life and symptoms caused by intracranial hypertension. Immediate evacuation can save the life of patients with subdural haematoma. Histopathological study can establish a diagnosis, especially in patients with known systemic primary disease.37,38 High doses of corticosteroids (dexamethasone) can cause temporary symptom relief.38 Whole brain radiotherapy can be used as palliative therapy if the dural lesions are associated with parenchymal metastases, while radiosurgery is reserved for isolated lesions less than 3cm in diameter.36,38
PrognosisThe prognosis of dural metastases can be extrapolated from the prognosis of brain metastases under 1 year of survival. Most patients die due to systemic disease complications before they die from neurological complications. Prognosis is most favourable for haematological neoplasms, breast and lung cancer, with a survival of 365, 273 and 120 days respectively.37,38
ConclusionsAlthough brain metastasis is not an uncommon condition worldwide, in Mexico there are no multidisciplinary programmes established to provide continuity for patients in whom the primary tumour has been identified, even though up to 40% of patients with systemic cancer have brain metastases. It should be noted that even after a thorough study, the primary tumour cannot be identified in 15% of brain metastasis patients. The most common types of neoplasm that present brain metastasis are lung cancer, breast cancer, melanoma, colorectal cancer and renal cancer. More reliable statistics must be established in Mexico in order to combat brain metastasis. Given the new therapy techniques (surgery, chemotherapy and radiotherapy) and increased survival, in patients in whom the primary tumour has been identified there is a possibility that the risk of brain metastasis may increase, and therefore continual review protocols must be diligently applied in these patients. This will help in the diagnosis and suitable management of these patients.
Conflict of interestThe authors declare that they have no conflict of interests.