Antecedentes: dada la frecuencia de procesos infecciosos en personas con el síndrome de Down, puede plantearse la necesidad de un estudio inmunitario.
Objetivo: comparar los valores de distintos parámetros de la inmunidad en un amplio grupo de pacientes, para conocer los porcentajes que se separan de los valores normales.
Material y métodos: en un total de 433 casos se valoró la IgG total, la IgA y la IgM y en menos casos también las IgG2 y las IgG4 o las IgG1 y las IgG3, así como los linfocitos T4/ T8 y el porcentaje T4/T8. En 85 de ellos se valoró también el nivel sérico de zinc. Los valores de las tres primeras inmunoglobulinas se han comparado con los correspondientes de 211 niños no afectados por el síndrome.
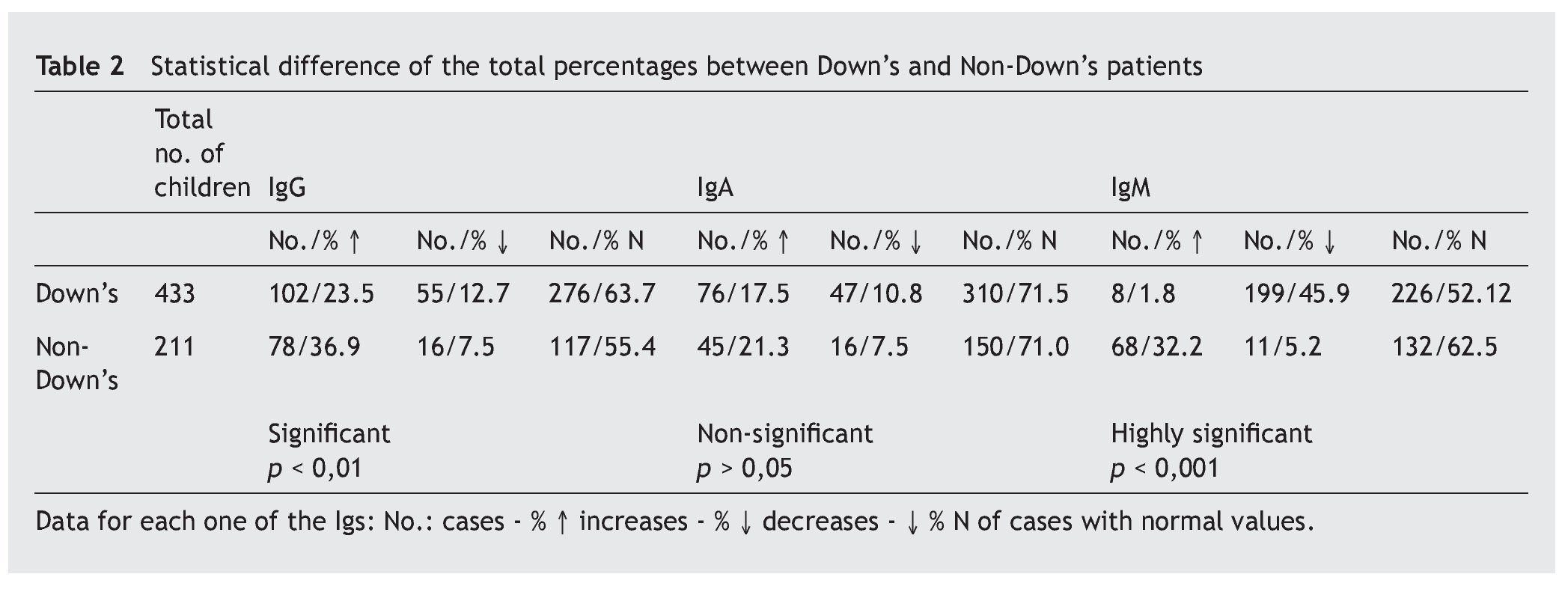
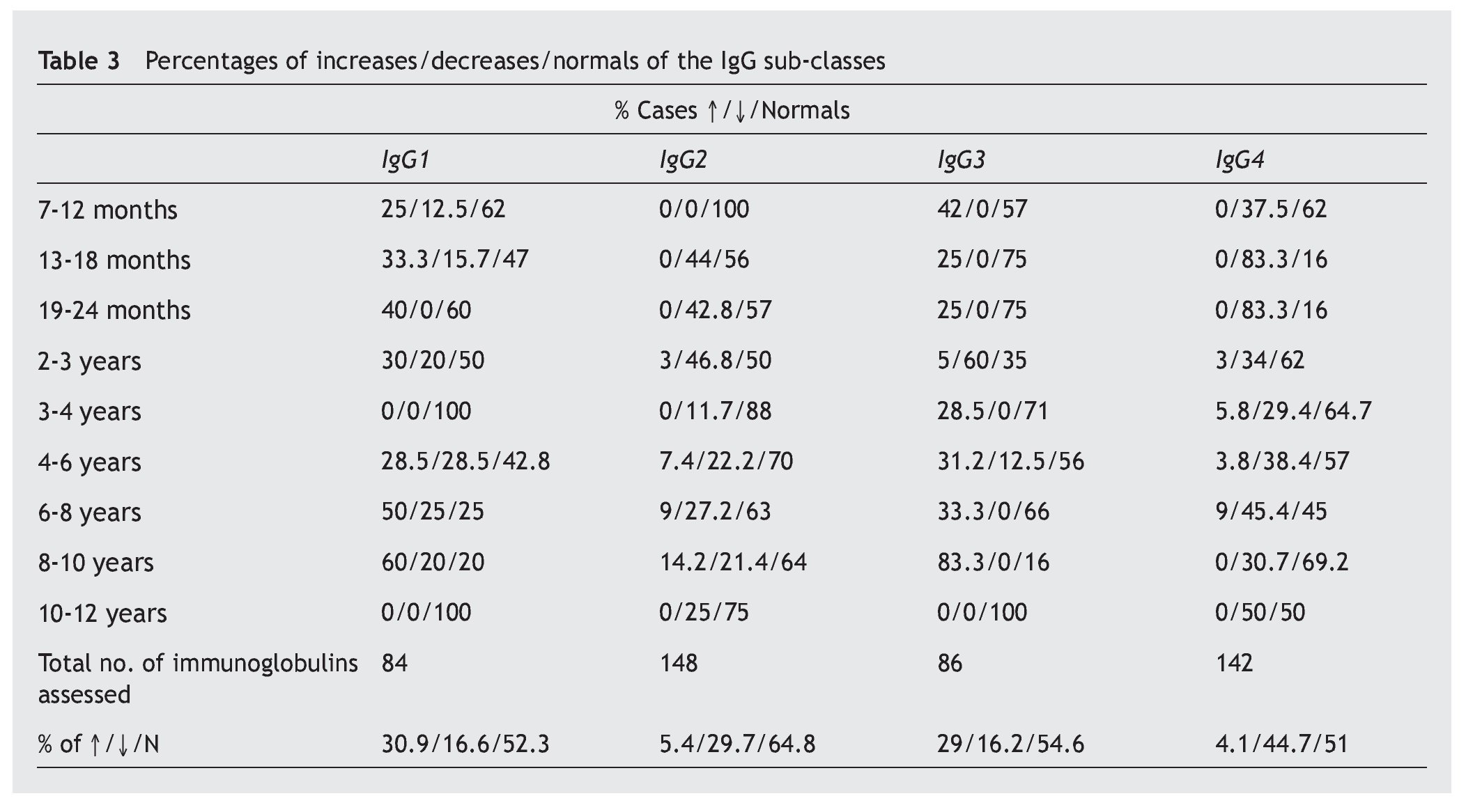
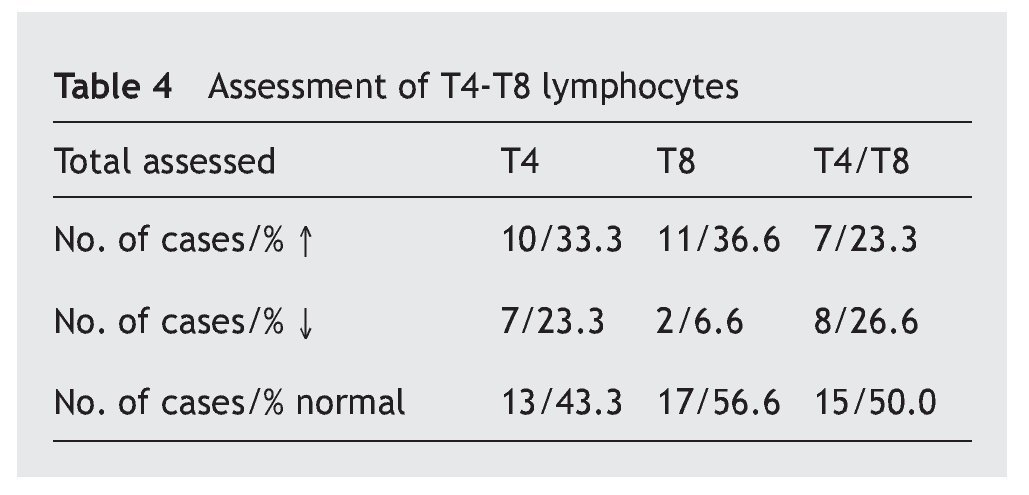
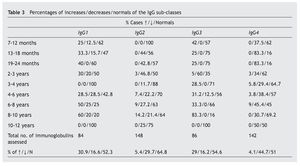
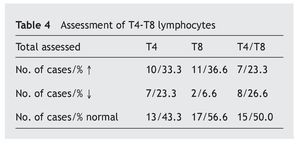
Resultados: los aumentos, disminuciones o valores normales de las IgG, IgA e IgM en comparación con los de individuos no afectados son significativos para IgG (p < 0,01), no significativos para IgA (p ≥ 0,05) y altamente significativos para IgM (p < 0,001). En las subclases de IgG predominan los valores normales, con aumentos de IgG1 e IgG3 (30,9% y 29%) superiores a IgG2 e IgG4 (5,4% y 4,1%) y disminución más significativa de IgG2 e IgG4 (29,7% y 44,7%) que para IgG1 e IgG3 (16,6% y 16,2%). Linfocitos: predominio de valores normales, con disminución del 23,3% de T4 y 6,6% de T8 y del 26,6% de T4/T8. En el 89,5% de los casos, los valores de zinc son normales, con un descenso en el 5,8%. Conclusiones: la frecuencia y/o gravedad de las infecciones obliga a efectuar un estudio de la inmunidad, inicialmente con los parámetros aquí estudiados, que deberán ampliarse cuando los valores se aparten excesivamente de la normalidad.
Background: Given the frequency of infectious processes in individuals with Down’s syndrome, the need for an immune study may arise.
Objective: To compare the values of certain immune parameters in a wide group of patients, and to determine the percentages that are outside the normal values.
Material and methods: A total of 433 cases were studied, in which total IgG, IgA and IgM were evaluated, and in fewer cases the IgG2 and IgG4 or IgG1 and IgG3, as well as T4 and T8 lymphocytes and T4/T8 ratio. In 85 of these cases the serum zinc level was also measured. The IgG, IgA and IgM values were compared with those of 211 children unaffected by the syndrome.
Results: The increased, decreased or normal values of IgG, IgA and IgM, in comparison with those with who do not have Down’s syndrome, are significant for IgG (p ≥ 0.05), non-significant for IgA (p ≥ 0.05), and highly significant for IgM (p < 0.001). In the IgG subclasses, normal values predominated, with increases in IgG1 and IgG3 (30.9% and 29%), higher IgG2 and IgG4 (5.4% and 4.1%), and a more significant decrease in IgG2 and IgG4 (29.7% and 44.7%) than for IgG1 and IgG3 (16.6% and 16.2%). Lymphocyte values showed more normal values, with 23.3% and 6.6% with a decrease in T4 and T8, respectively, and 26.6% with a decreased T4/T8. The zinc values are normal in 89.5% of the cases, with a decrease in 5.8%.
Conclusions: The frequency and severity of the infections makes it obligatory to carry out an immune study, which must be extended when the values are excessively outside the normal ranges.
Introduction
Among the different, not infrequent, risks in patients with Down’s syndrome (DS), infections occupy an important place, with those affecting the respiratory system or ears being more predominant. It is known that there are several variants of different elements in these children that take part in the immune response, in particular the lymphocytes and immunoglobulins.
The aim of this work is to evaluate the blood levels of the immunoglobulins IgG, IgA and IgM, CD4 (T4) and CD8 (T8) lymphocytes, and zinc, which have been associated with the immune response, based on the values obtained for these parameters on patients studied by paediatricians of the Catalan Down’s Syndrome Foundation (FCSD).
Material and Methods
The total IgG was evaluated in 433 patients between 8 months and 18 years, plus IgG2 and IgG4 in many of them, and also IgG1 and IgG3 in fewer cases. CD4 and CD8 lymphocytes were assessed in 30 cases, as well as the ratio between them, and the serum zinc levels in 85 cases, with seven children, assessed with a different parameter, not having been included.
The total IgG, IgA and IgM were compared with those of another 211 children with the same age range but not affected by the syndrome, and the majority of them with suspicion or confirmation of an atopic disease.
A study carried out on Spanish children between 7 months and over 14 years old in order to determine normal values of the different immunoglobulins (total IgG and sub-classes, IgA and IgM) has been used to compare their values with those of the children in the study1.
The Mann-Whitney U-test has been used to compare the IgG, IgA and IgM between DS and non-DS patients.
The reference values for the T4 and T8 lymphocytes have been estimated from the study on European children by Bunders et al.2.
The assessment of the reference values of serum zinc in μg/dL has been based on the work by Papadopol3.
All the quoted normal values are those endorsed by the laboratories that performed the different measurements on the children of the study.
Results
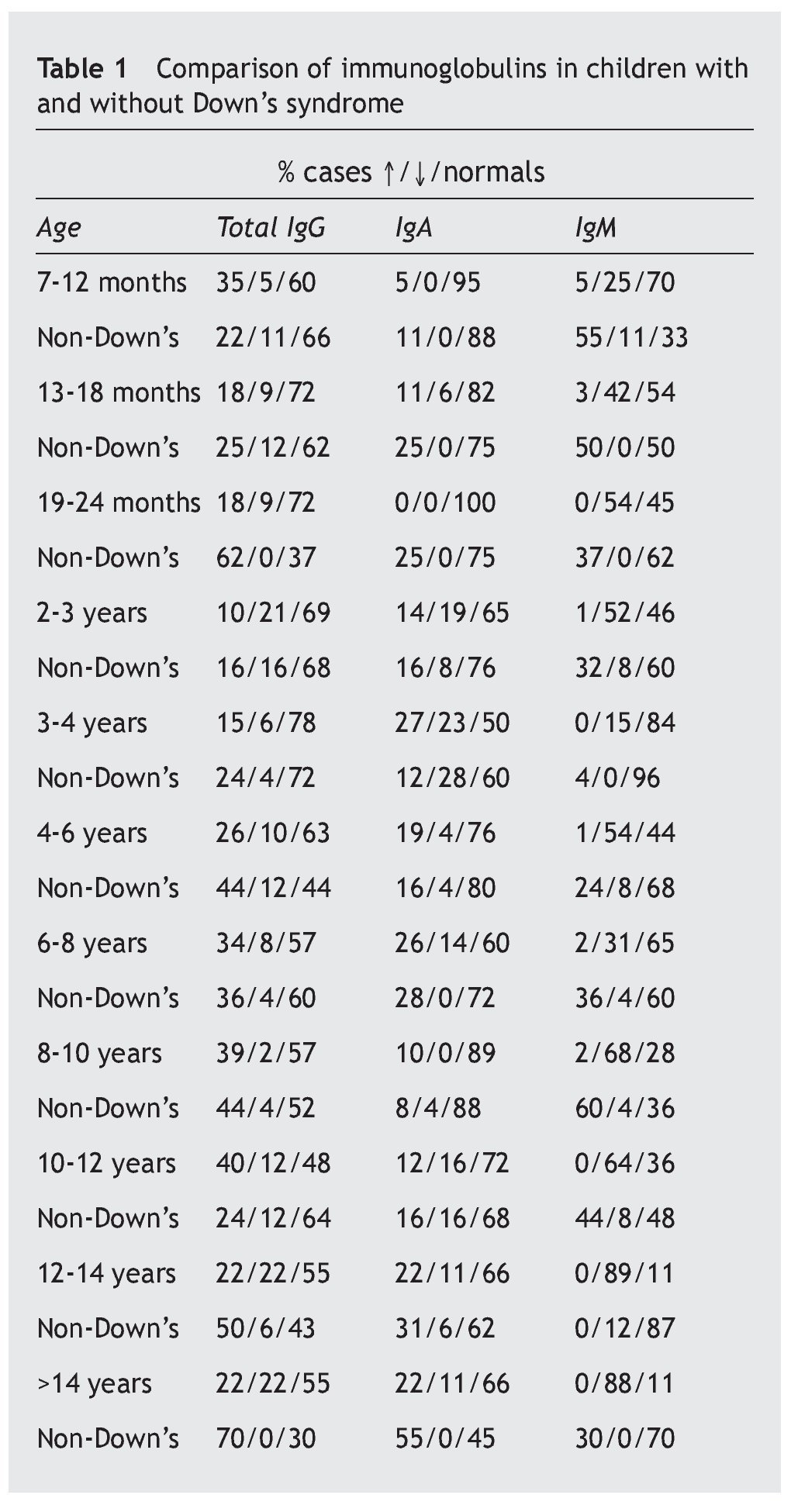
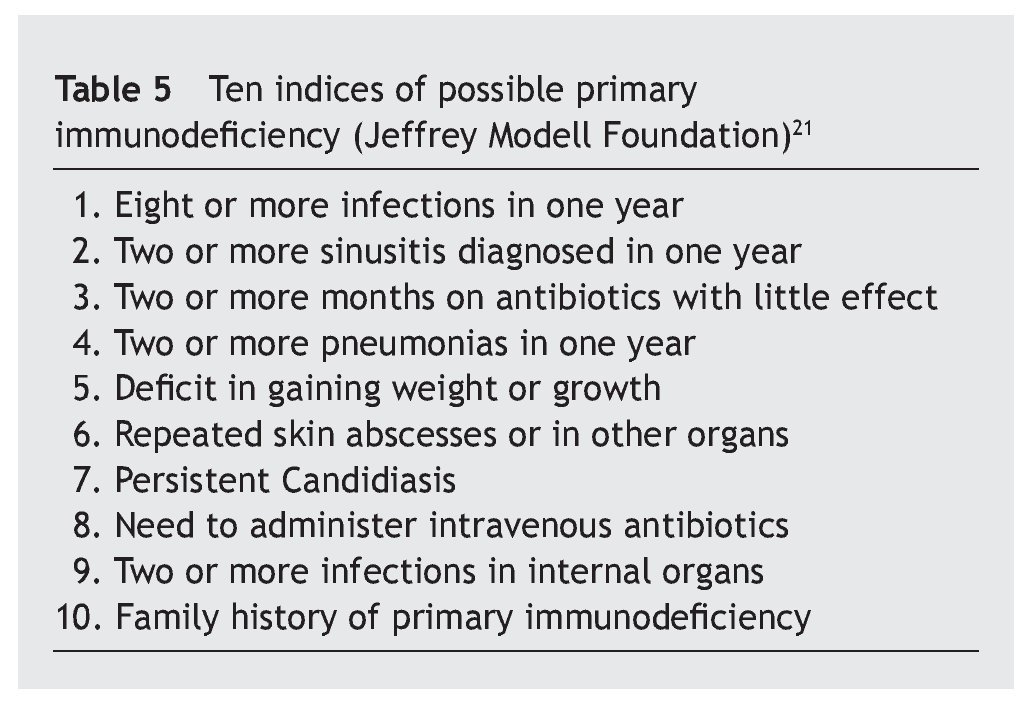
Table 1 shows the number of cases and the percentage with increases, decreases and normal in each of the three immunoglobulins (total IgG, IgA, IgM) in the patients, and the comparison of each one of these parameters with the children who did not suffer from the syndrome.
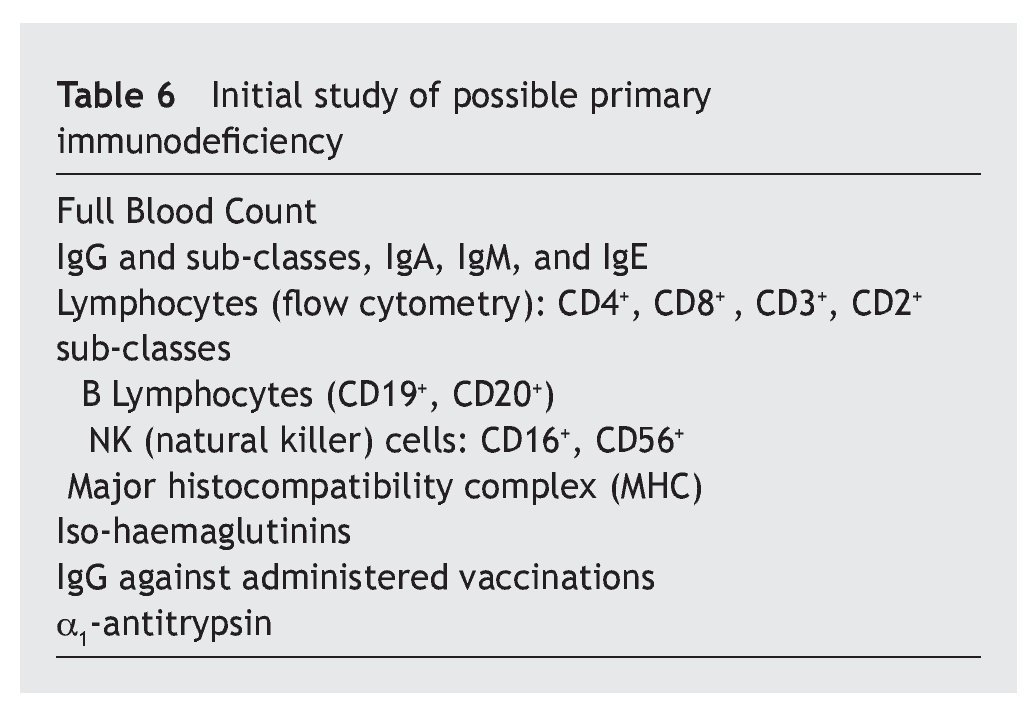
Overall, it is observed that the difference for IgG is significant (p < 0.01), and very significant for IgM (p < 0.001), but is not the case for IgA (p ≥ 0.05) (table 2). Even so, a lower percentage of cases with increased values for IgG is observed in the patients, at the same time that there is a greater number of decreases. There are no significant differences for IgA, while there are marked differences for IgM, which show a notable difference in the decreased values, with few cases that have increased values.
A notable decrease from the normal value is observed in the IgG sub-classes, in IgG4 (44.7%), and in a lower proportion, in IgG2 (29.7%), while the most notable increases are in IgG1 (30.9%) and IgG3 (29%) (table 3).
In table 4 it is observed that a major proportion of both sub-classes of lymphocytes, T4 and T8 (also called CD4 and CD8), have normal values (43.3% and 56.6%, respectively), while the increase in the values is similar (33.3% and 36.6%) and the decrease in the T4 is greater (23.3% compared to 66.6%). The T4/T8 ratio is normal in half of the cases, with the percentage increases and decreases being similar.
The serum zinc levels were normal in 89.5% of the cases, increased in 4.7%, and decreased in 5.8%.
Discussion and comments
IgM is the principal and primary immunoglobulin of the immune response against diverse infectious agents, while IgG is the major component of the secondary response, and the two of them take part in the activation of the complement system. These data could be associated with the higher incidence of infectious processes in the patients studied, given the notable decreases in their values, particularly IgM. On the other hand, the increase in IgG may mean a good response against pathogens. The number of normal values of serum IgA is explained by its main function being developed in the mucosal tissues (IgA1, IgA2, secretory IgA).
As regards the IgG subclasses, there are notable decreases in IgG2 and IgG4, which have a more active presence against polysaccharide antigens (e.g., pneumococcal, Haemophilus influenzae), while the IgG1 and IgG3 act against protein antigens (e.g., diphtheria, tetanus)4,5. Deficiencies in these sub-classes have generally been associated with the higher frequency of recurrent infections in these patients, although they are deficiency variables that do not coincide with any of the catalogued immunodeficiencies, unlike the other components of the immune system included in this study6,7.
The T lymphocytes are divided into two main types that have functions with different effects depending on the expression CD4 and CD8 proteins on their surface (T4 and T8). Antibody production is a result of the activity of these lymphocytes that are known, respectively, as helper and suppressor, or cytotoxic. The harmful antigens (different germs) captured by the dendritic cells stimulate either antibody production (IgG specific) by the B lymphocytes due to the action of the T4 cells, or by viral suppression or by malignant cells by the T8 cells. For an increased response, the normal ratio between them both is 2/14,8. Therefore, the greater decrease of T4 in this study (23.3% compared to 6.6% T8) may also be associated with the incidence of infectious processes in these patients.
As can be seen, the zinc values obtained in this study hardly vary from normal, unlike the other elements that clearly have a marked importance in the immune defence mechanisms.
A result of these changes in immune parameters is the high incidence of infectious processes, among those that predominate are those that affect the respiratory system, including pneumonia, bronchiolitis, croup, acute respiratory distress syndrome (ARDS), infections that can give rise to septicaemia, which increases the mortality risk. On the other hand respiratory syncytial virus (RSV) has been shown to be more aggressive in these patients. In second place there is otitis media, and less frequently hepatitis A and B, gingivitis, conjunctivitis, and cystitis9-11.
Although it is thought that the different organic anomalies, whether of the ear, respiratory tract, or gastrointestinal reflux, may contribute to the incidence of infections, the changes in the immune response are the main cause of these. The reasons for this deficiency have still not been absolutely determined, although importance is given to the lower number of T lymphocytes produced by the thymus, a gland which is smaller in these children compared to the normal population. This could result in lower activity of the T4 and T8 lymphocytes and the consequent reduction in immunoglobulins12-14.
Zinc is another element involved in the regulation of T lymphocytes, through several mechanisms that are still not fully understood, but without a doubt, its deficiency causes an imbalance in the Th1/Th2 lymphocytes ratio due the decrease in the hormonal activity of the thymus11,15,16. Zinc deficiency is associated with malnutrition, but it should be taken into account that serum zinc levels start to decrease from 5 years onwards and excessive intake may cause anemia, delayed growth, copper deficiency, and even immune impairment12.
With DS being due to a genetic alteration, without a doubt some genes of chromosome 21 must play a decisive role in the changes in immune response. Although it is not absolutely clear in which genes this deficiency may be found, on the basis of having evaluated the over-expression of two of them, called SOD1 and ITGB2, and perhaps also the calcineurin regulator (RCAN1), which is a transcription factor that influences lymphocyte T activation, although it cannot be ruled out that other genes may be involved11,17.
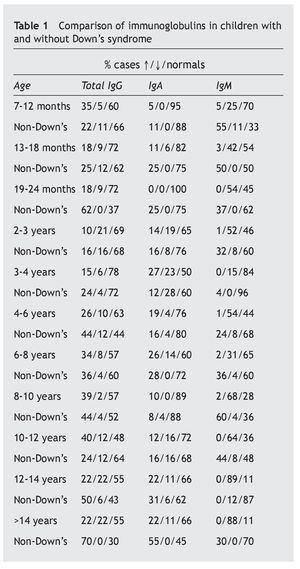
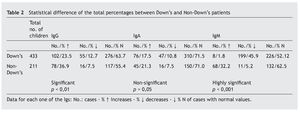
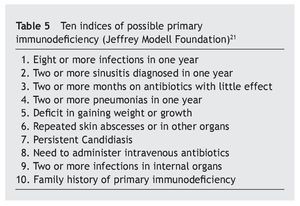
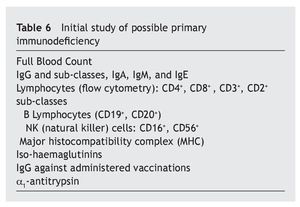
In any case, these immune alterations must not be overvalued, although they are really the basis of sporadic infections, with different frequencies and severity, which as has been seen, do not affect all patients equally, that have relatively acceptable normal values. Therefore, in accordance with Gámez-González, “it is not considered a primary immune deficiency, but more a syndromic deficiency due to the importance of the characteristic neurological impairment”, but that also “it could be placed among the classifications of a disease with thymus dysfunction”18. It is different from that occurring with the most important immunodeficiencies classified as primary, and have genetic bases situated in other chromosomes4,19,20, although it is possible that some DS patients may have both anomalies at the same time. In this review, only three of the children were found to have immunoglobulin levels compatible with primary immunodeficiency, which ruled out extending the immune study. In this sense, it is worth drawing attention to this possibility, as it may be useful to have a series of criteria that would suggest the need to extend the immune study (table 5)21 that initially must be focused on the assessment of the different elements of the immune response (table 6)22.
Conclusions
In patients affected by DS, the assessment of the different elements of the immune defence is especially indicated when the infectious processes are repeated and severe. When there are significant deficiencies in an initial evaluation, a complete immunological study should be made to see if there is a well catalogued immunodeficiency associated with changes in the usual immunity in DS.
Received on July 20, 2014;
accepted September 18, 2014
*Author for correspondence.
E-mail:5314fml@comb.cat (F. Muñoz-López).