Evaluate the shear bond strength of a self-etching system to enamel pretreated with ozone and its type of fracture.
Matherial and methodsThirty sound bovine incisors were bisected and polished just before the application of the adhesive system. The adhesion area was limited to a 3-mm diameter. The specimens were randomly assigned to the experimental groups (n=15) and composite resin cylinders were added to the tested surfaces, after the application of the adhesive according to the manufacturer's instructions. Group G1 (AdheSE® with ozone) was previously prepared with ozone gas from the HealOzone unit (Kavo®) for 20s, groups G2 (AdheSE®) was used as control. The specimens were stored in distilled water for 24h at 37°C with 100% humidity, before being thermocycled. The type of fracture was analyzed under scanning electronic microscope and the data were submitted to Shapiro–Wilk, Student's t-test and Chi-squared statistical analyses.
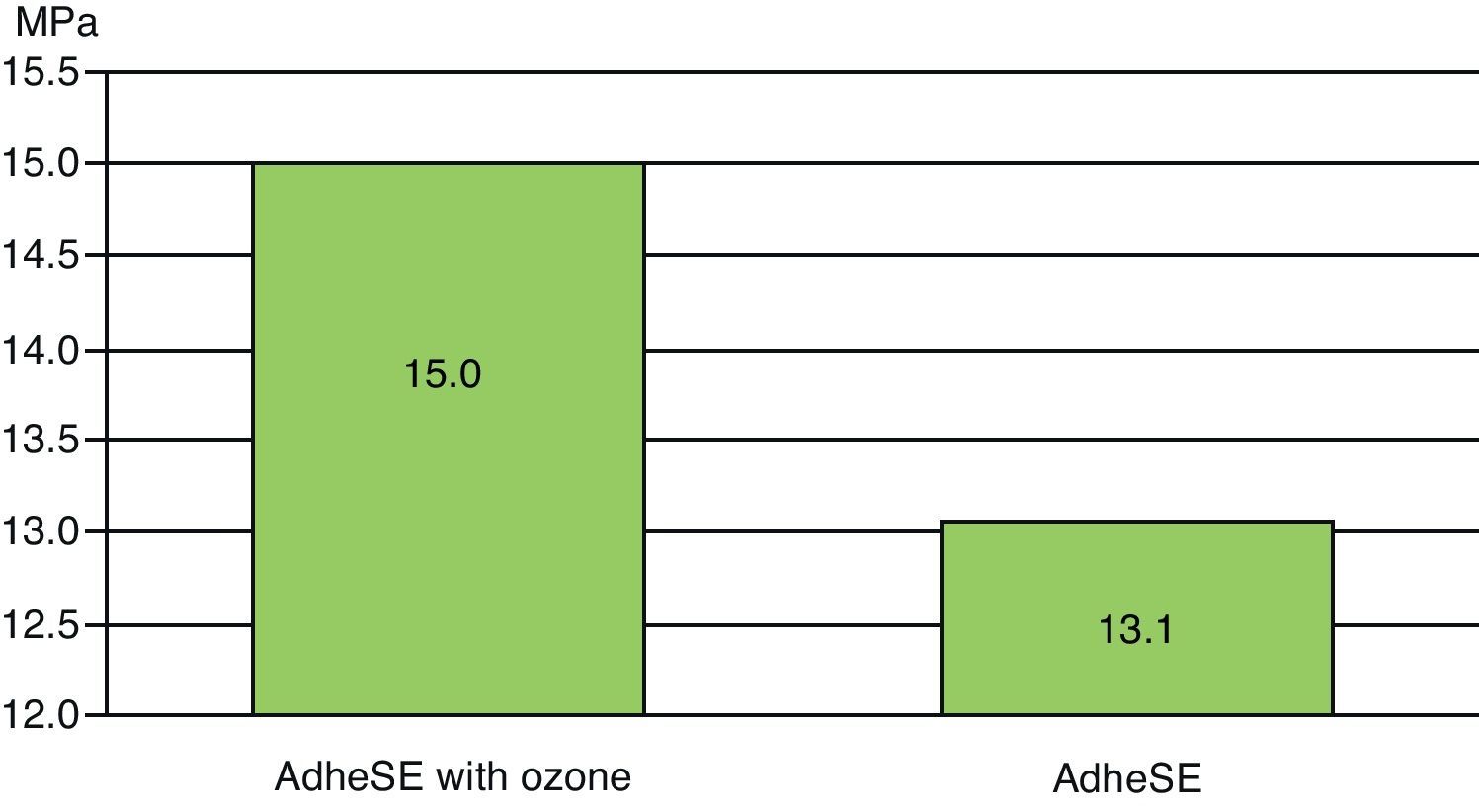
ResultsThe mean bond strengths were G1: 15.0MPa (77.8% of adhesive fractures between enamel and adhesive) and G2: 13.1MPa (36.4% of adhesive fractures between enamel and adhesive).
ConclusionThe shear bond strength of a self-etching system was not influenced by the previous application of ozone gas.
Avaliar as forças de resistência adesiva de um sistema adesivo auto-condicionador no esmalte pré-tratado com ozono e o tipo de fractura.
Material e métodosTrinta incisivos hígidos de origem bovina foram seccionados de forma a separar a coroa da raíz e polidos antes da colocação do sistema adesivo. A área de adesão foi limitada a 3mm de diâmetro. Os espécimes foram aleatoriamente divididos (n=15) e cilindros de resina composta foram adicionados às superfícies de teste após cada sistema adesivo ter sido aplicado de acordo com as instruções do fabricante. O grupo G1 (AdheSE® com ozono) foi condicionado com gás de ozono gerado pelo aparelho HealOzone (Kavo®), durante 20 segundos, G2 (AdheSE®) funcionou como controlo. Os espécimes foram mantidos em água destilada durante 24 horas numa estufa a 37°C com 100% de humidade, antes da termociclagem. O tipo de fractura analisado ao MEV e os dados submetidos à análise estatística Shapiro–Wilk, Student's t-test e Chi-squared.
ResultadosAs médias de resistência adesiva foram: G1:15,0MPa (77,8% de fracturas adesivas entre o esmalte e o adesivo) e G4: 13,1MPa (36,4% de fracturas adesivas entre o esmalte e o adesivo).
ConclusãoOs valores de resistência adesiva do sistema adesivo auto-condicionador não foram influenciados pela aplicação prévia de gás de ozono.
Currently, it is not possible to assure that a tooth cavity is bacteriologically aseptic, thus an antibacterial treatment of the dental surface previous to restoration has been advised.1 Indeed, some authors have started to apply Ozone as a disinfecting agent.2 Ozone, with its antibacterial action due to its strong oxidizing activity, is an important disinfecting agent.3–5
Recent research reveals the bactericidal action of ozone against Streptococcus mutans and other bacteria commonly found in cervical caries.5,6 However, there are very few data concerning its effect on dental adhesion.7 Previous studies demonstrated that oxygen and other oxidant agents (such as whitening agents) have a negative influence on bond strength values of dental-enamel adhesives.
Resin–enamel adhesion is one of the most significant advances in the history of Dentistry8 and it is used in our days as a simple effective procedure, when using a total-etch technique.9 Nevertheless, the enamel etching concept has been improved through the years and new adhesive systems have been developed and released.10,11
Self-etching systems were developed to simplify and eliminate some of the clinical steps associated to total-etch.11 Self-etching adhesives are based on acidic monomers that simultaneously condition and prime enamel.12 The primer is applied on the enamel and resin tags are form. Smear layer is dissolved and incorporated into the bonding process, therefore the tooth no longer requires rinsing, as it does with etch-and-rinse.13,14
One of the questions that arise is whether the acidic monomer used in self-etch adhesive systems is capable of promoting enamel demineralization, making it a reliable and durable adhesion.15,16 Shear bond strength tests aim to establish a numeric value in order to determine how strong that bond is.17 In addition, since no rinsing occurs after the application of the self-etching, we may speculate that self-etch systems are more susceptible to Ozone residual oxygen.
This study aimed to determine whether ozone gas is safe to use in bovine enamel regarding its effect on Shear Bond Strength (SBS) when using a self-etching adhesive (AdheSE®, Ivoclar vivadent AG, Liechtenstein).
Materials and methodsThirty sound bovine incisors were extracted for no longer than a month and kept in distilled water at 4°C. After this period of time, the teeth were kept in a 0.5% chloramine solution for a week and bisected with a microtomer (Accuton-Struers, Copenhagen, Denmark) to separate the crown from the root. They were then polished with a 240-grit sandpaper to create a flat surface and polished, again, with a 320-grit sandpaper (Carbimet Buehler-met, Buehler, Lake Bluff, IL) to simulate the smear layer just before the application of the adhesive system. Polyester film (Mylar, Dupont Corp., DE, USA), with a 3-mm diameter hole was used to restrict the adhesion area. Specimens were randomly assigned to one of two experimental groups (n=15) and composite resin cylinders were bonded to the tested surfaces, after the application of the adhesive according to the manufacturer's instructions: AdheSE Primer was applied with a brush. Once the surface was completely coated, the primer was brushed into the entire surface for another 15s. The total reaction time was not shorter than 30s. The primer was dispersed with a strong stream of air until the mobile liquid was no longer visible. Then, AdheSE Bond was applied and dispersed with a weak stream of air and polymerized for 10s. Group G1: AdheSE® with ozone (Ivoclar vivadent AG, Liechtenstein) was conditioned for 20seconds with ozone gas from the HealOzone unit (Kavo®, Germany) using a 5-mm delivery cup (green). Groups G2 (AdheSE®) was used as control, not receiving ozone before the application of the adhesive system. The adhesive materials used in this study are listed in Table 1 along with the manufacturers’ compositions, batch numbers and codes. After this application, specimens were kept in distilled water for 24h at 37°C with 100% humidity (Hemmet, Schwabach, Germany) in order to obtain the maximum resin polymerization, before being thermocycled (Aralab, mod 200E, Cascais, Portugal) for 500 cycles at 5° and 55°C for 20s18 in each bath and submitted to shear testing at a crosshead speed of 0.5mm/min (Instron, model 4502, series H3307, Instron Ltd, Bucks, England). The type of fracture was analyzed under SEM (JEOL JSM 6301F, Tokyo, Japan). Fractures were classified by a single experienced investigator, as either adhesive, cohesive (resin or enamel) or mixed19 and the data were submitted to Shapiro–Wilk, to evaluate the normality and Student's t-test to compare both groups. Chi-squared statistical analyses were used to compare the type of fractures.
Restorative and adhesive materials.
| Materials | Function | Composition | Batch# |
| AdheSE® (lvoclar vivadent A G, Liechtenstein) | Adhesive system | Primer: Mixture of dimethacrylate, phosphonic acid acrylate, water, initiators and stabilizers; Bond: Bis-GMA, HEMA, GMDA, | J03385 |
| Synergy D6 (Coltene whaledent GmbH+Co. KG Germany) | Restorative material | Methacrylates, barium glass silanized, amorphous silica | 0145721 |
The mean Shear Bond Strengths (SBS) shown in Fig. 1 were G1: 15.0MPa (77.8% of adhesive fractures between enamel and adhesive) and G2: 13.1MPa (36.4% of adhesive fractures between enamel and adhesive). A typical fracture between enamel and adhesive can be observed in Fig. 3.
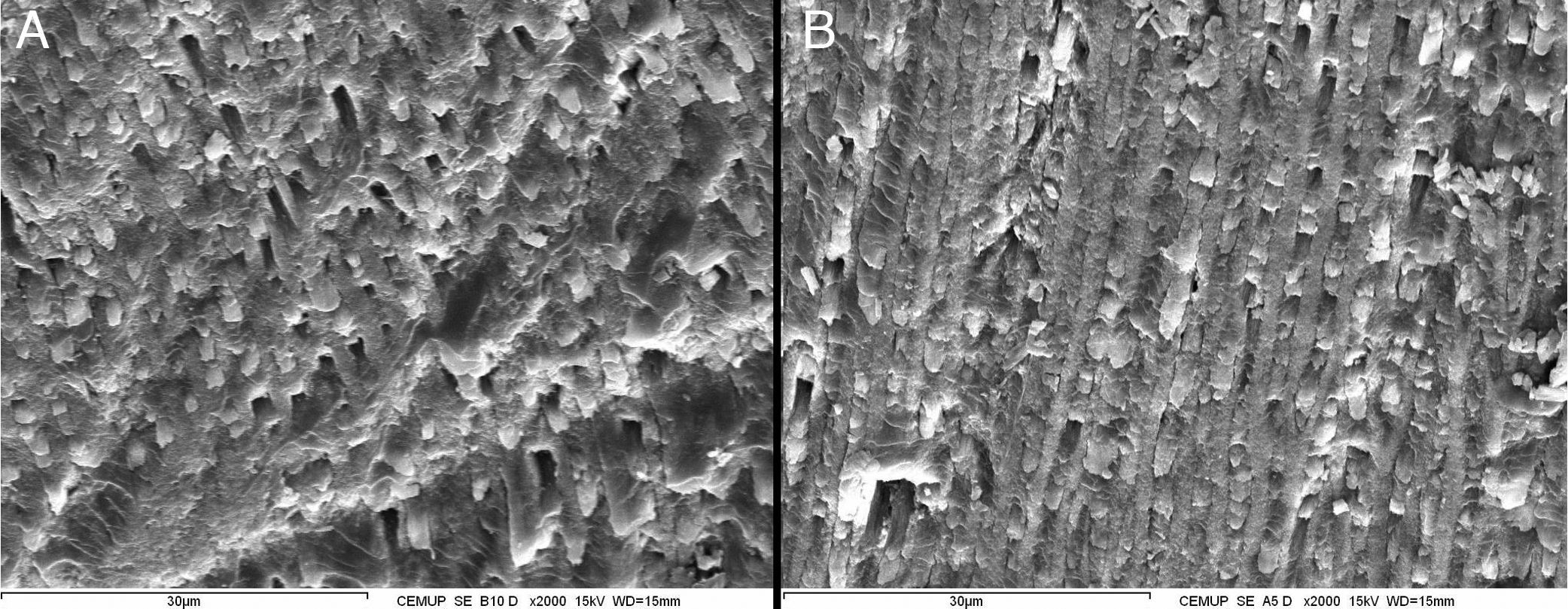
No statistic differences were found between G1 and G2 (p>0.05). The enamel surface obtained with and without ozone can be seen in Fig. 2.
DiscussionIn the present study, AdheSE® mean SBS values did not differ significantly between Ozone pretreated group and the control group (15.0MPa and 13.1MPa with Ozone pretreatment and control, respectively).
The result of this work seems to show no harm influence of ozone in terms of Shear Bond Strength.
In this study, ozone was applied on enamel for 20s. Ozone eliminates 99.9% of bacteria present after this period.2,3,20,21 This very short ozone gas application time when compared with other potential oxidants, like bleaching agents, may explain why ozone gas did not decreased SBS values in the present study, as it was expected.22,23
Consistent with our results, Schmidlin et al.7 found no statistical differences on SBS values when a total-etch system was used. In a study conducted in February 2008,24 SBS values of orthodontic brackets to enamel had no differences in groups treated with or without ozone. The same results were obtained in a contemporaneous study.22
Ozone is a promising alternative in modern Dentistry. The application of ozone to enamel may be important to disinfect the surface before applying sealants or orthodontic brackets. Recent studies shown its ability to provide an antibacterial treatment against S. mutans even after 8 weeks.25 The current work complements a growing body of work demonstrating the safe usage of ozone in dental adhesion.7,22,24
Investigation focusing on the long-term effects is recommended for further research.
AdheSE® is a two-step self-etching adhesive developed with the expectation of decreasing the time and technical sensitivity of the adhesion process. It is intended that they will substitute the total-etching adhesives, as manufacturers claim that these products have a similar behavior and are easier to work with.26 The main concern about self-etch materials is the fact that they may not be able to etch efficiently tooth enamel.16 The results of published studies on the efficacy of bonding to enamel are not pacific,27 on the contrary to etch and rinse; this was the reason for using a self-etching system in our study.
The results of AdheSE® SBS values in enamel were low, however, our findings are consistent with other several studies.11,26,28,29 Some authors state that bond strength of self-etch adhesives is not as good as adhesive systems that use total etching with phosphoric acid.30
The main reason might be the total-etch acidic capacity of demineralizing enamel better and deeper than self-etch adhesives.
However, Hannig et al.31 using self-etching adhesives, found similar SBS with or without pre-etching enamel showing that a better and deeper demineralization might not mean higher SBS values. Other researchers have shown that although demineralization patterns are not as accentuated as self-etch adhesives, they reach highly satisfactory levels of bond strength.11,32,33
Self-etching adhesives are less susceptible to the operative procedures and might show similar results in vivo and in vitro, which may not be true for total-etching adhesives that are more susceptible to the technique.
Concerning the type of fracture, it was not possible to withdraw any conclusions in our study, since the differences were not statistically significant.
ConclusionsGaseous ozone caused no reduction in Shear Bond Strength values in enamel when self-etch systems were used. Self-etching systems were not influenced by the previous application of ozone gas in shear bond strength.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.