Auditory hallucinations (AH) are one of the most prevalent symptoms of schizophrenia. They might cause several brain alterations, especially changes in the volumes of hippocampus and amygdala, regions related to the relay and processing of auditory cues and emotional memories.
Material and methodsWe have recruited 41 patients with schizophrenia and persistent AH, 35 patients without AH, and 55 healthy controls. Using their MRIs, we have performed semiautomatic segmentations of the hippocampus and amygdala using Freesurfer. We have also performed bilateral correlations between the total PSYRATS score and the volumes of affected subregions and nuclei.
ResultsIn the hippocampus, we found bilateral increases in the volume of its hippocampal fissure and decreases in the right fimbria in patients with and without AH. The volume of the right hippocampal tail and left head of the granule cell layer from the dentate gyrus were decreased in patients with AH. In the amygdala, we found its left total volume was shrunk, and there was a decrease of its left accessory basal nucleus in patients with AH.
ConclusionsWe have detected volume alterations of different limbic structures likely due to the presence of AH. The volumes of the right hippocampal tail and left head of the granule cell layer from the dentate gyrus, and total volume of the amygdala and its accessory basal nucleus, were only affected in patients with AH. Bilateral volume alterations in the hippocampal fissure and right fimbria seem inherent of schizophrenia and due to traits not contemplated in our research.
Las alucinaciones auditivas (AH) son uno de los síntomas más prevalentes de la esquizofrenia. Pueden causar varias alteraciones cerebrales, especialmente cambios en los volúmenes del hipocampo y de la amígdala, regiones relacionadas con la transmisión y el procesamiento de señales auditivas y recuerdos emocionales.
Material y métodosHemos reclutado 41 pacientes con esquizofrenia y AH persistentes, 35 pacientes sin AH y 55 controles sanos. Usando sus resonancias magnéticas, hemos realizado segmentaciones semiautomáticas del hipocampo y de la amígdala usando Freesurfer. También hemos realizado correlaciones bilaterales entre la puntuación total de PSYRATS y los volúmenes de subregiones y núcleos afectados.
ResultadosEn el hipocampo encontramos aumentos bilaterales en el volumen de la fisura hipocampal y disminuciones en la fimbria derecha en pacientes con y sin AH. El volumen de la cola del hipocampo derecho y la cabeza izquierda de la capa de células granulares del giro dentado se redujeron en pacientes con AH. En la amígdala, encontramos que su volumen total izquierdo estaba reducido y había una disminución de su núcleo basal accesorio izquierdo en pacientes con AH.
ConclusionesHemos detectado alteraciones en el volumen de diferentes estructuras límbicas, probablemente debidas a la presencia de AH. Los volúmenes de la cola del hipocampo derecho y la cabeza izquierda de la capa granular del giro dentado, y el volumen total de la amígdala y su núcleo basal accesorio, solo se vieron afectados en pacientes con AH. Las alteraciones bilaterales de volumen en la fisura del hipocampo y la fimbria derecha parecen inherentes a la esquizofrenia y debidas a rasgos no contemplados en nuestra investigación.
Around 75% of patients with schizophrenia also present auditory hallucinations (AH).1 This positive symptom can usually be controlled using pharmacologic treatments, but in some cases it becomes persistent. Although we still do not know the extent of the causes that are behind this symptomatology, a widespread hypothesis explains it might be caused by alterations in the limbic system.2 Furthermore, a later subsequent hypothesis state that changes in this system are more likely responsible for the positive symptomatology.3
Two of the main regions of the limbic system are the hippocampus and the amygdala, both located in the temporal lobe. The former relays cognitive information to the neocortex and is responsible for spatial memory, among other processes. It is formed by the Ammon's horn (CA), which can be segmented into a “head”, a “body” and a “tail”. The CA is also divided into different regions (CA1–CA4), being CA4 its distal part, which is inserted into the hilus of the dentate gyrus (DG). The DG is composed by different layers, including the granule cell layer, composed by the somata of the granule cells and the molecular layer, which contains the apical dendrites of these neurons. The DG is separated from the CA and the subiculum by the hippocampal fissure, which is an expansion of the lateral ventricles.4 The hippocampus receives and sends reciprocal information from subcortical structures through the fimbria,5 which becomes the fornix once it emerges from the hippocampus.4 On the other hand, the amygdala is commonly known for storing emotional memories and processing fear stimuli.6 It is habitually split into different nuclei, including the central, lateral, basal, and accessory basal nuclei, which display different specific functions and connectivity.7
There is much evidence on the alterations of the hippocampus and amygdala in schizophrenia, although these studies oftentimes merge these two regions due to their proximity. Several reports that analyzed MRIs from chronic patients with schizophrenia concluded that they presented reduced bilateral volumes of the hippocampus–amygdala complex8,9; interestingly, the volume of this complex was inversely correlated with symptom severity.10
With the emergence of new neuroimaging and segmentation techniques, several reports have been able to estimate the volume of different hippocampal subfields. Some of these studies showed that the volume loss was only significant on the left hippocampus in chronic patients, who also displayed smaller left CA2-CA4 and DG,11 while in other cohort of similar individuals these decreases were bilateral.12 Interestingly, some of these structural changes were already detectable in first episode patients, who showed enlarged bilateral hippocampal fissures.13 Furthermore, another segmentation study showed that a general cohort of patients (with no information on the duration of illness) had smaller bilateral hippocampi, presubiculum and DG molecular layer, as well as a reduced volume of the left subiculum, CA1 and hippocampal tail.14 In addition, a cohort of chronic patients displayed a smaller granule cell layer of the DG,15 an alteration that has recently been proposed as a critical factor in the etiopathology of this disorder.16 Another interesting report has described that patients with schizophrenia and a history of violence also had smaller bilateral hippocampi, and showed decreases in the volumes of CA1, molecular layer and fimbria, as well as an increase in the volume of the hippocampal fissure.17 Yet, all these alterations could be overshadowed by the chronic administration of typical and atypical antipsychotics, since these drugs may be modulating differentially the volume loss reported in schizophrenia.18
By contrast with the hippocampus, studies on the amygdala have rendered contradicting results. The study of Tesli et al. (2010) on patients with schizophrenia with a history of violence showed that they had a decreased bilateral volume of the amygdala, similar to what was described previously in another study analyzing a general cohort of this disorder.19 Recent analyses using finer segmentation tools have shown significant reductions in the volumes of the left amygdala and its lateral nucleus, as well as bilateral decreases in the basal and paralaminar nuclei and anterior amygdaloid area.14 A similar study has found reductions in most amygdaloid nuclei, including the accessory basal nucleus.20
These previous studies on the changes of hippocampal and amygdaloid volumetry in schizophrenia used general cohorts of patients, but very few of them focus on specific symptomatology, particularly the presence of AH. The information that emerges from this research can offer a deeper insight on the impact of the disease on the brain and particularly on the effect of persistent AH in areas relevant for fear and anxiety, such as the hippocampus and the amygdala. For instance, a study analyzing computerized tomography found that the width of the third ventricle was positively correlated to the frequency of AH.21 Specifically in the hippocampus, an MRI study using manual segmentation procedures showed that the volume of the right and left hippocampal tail was reduced in patients with schizophrenia and persistent AH.22 Regarding the amygdala, our laboratory found that its gray matter from the left hemisphere was reduced in patients with persistent AH.23 A similar reduction in whole amygdaloid volume was also described specifically in men with schizophrenia and AH.24
The PSYRATS scales are highly valuable to investigate different dimensions of AH and delusions.25 Work from our laboratory has shown that the total PSYRATS score in patients with AH was inversely correlated to the gray matter concentration of the right posterior and left inferior frontal gyri,23 and to the middle and superior temporal gyri.26 We have recently reported that the volume of different thalamic nuclei was inversely correlated to the total PSYRATS score in the same cohort of patients with schizophrenia and persistent AH that we are using in the present study.27 In addition, a study of resting state functional MRI has also demonstrated that the component of the posterior cingulate/temporal network, which includes hippocampus and amygdala, was inversely correlated to the set of emotional items from PSYRATS in patients with AH, a correlation that was not present in patients without AH.28
In this article we have gathered the volumes of the right and left hippocampus and amygdala from patients of chronic schizophrenia with and without persistent AH. After their segmentation with the use of probabilistic atlases, we have studied in detail the volume of their subfields and nuclei and have also correlated them with the total score from the PSYRATS scales.
Material and methodsStudy participantsAll the samples from both healthy subjects and patients suffering from schizophrenia come from two centers of the network of biomedical research centers in mental health (CIBERSAM): the Institute of research of the Clinic Hospital from Valencia (INCLIVA) and the Sant Pau Hospital from Barcelona. All the patients meet DSM-IV criteria for schizophrenia and reported experiencing AH, and all of them were able to read, understand and give an informed consent. None of them were hospitalized at the moment of evaluation, and all of them were legally competent. All the procedures were approved by the local Ethics Committee.
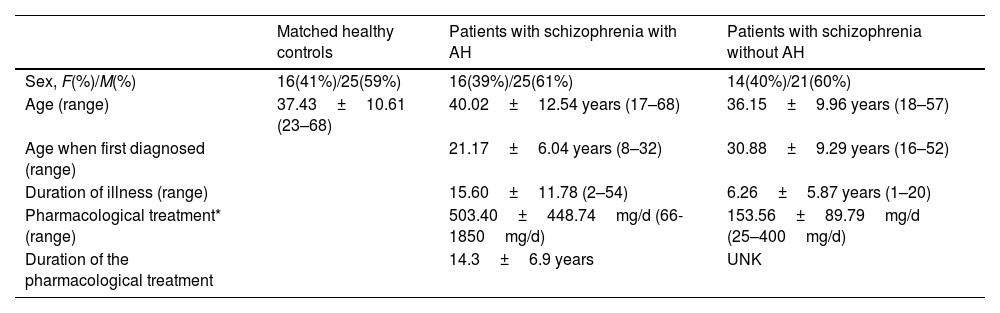
Three cohorts of subjects were considered in these analyses: A group of 41 patients diagnosed with schizophrenia in a chronic stage experiencing persistent AH, a group of 35 patients diagnosed with schizophrenia without AH, and 55 healthy control subjects. The inclusion criteria are summarized in supplementary methods, and its demographic traits can be found in Table 1.
Demographic and clinical data of patients with schizophrenia with and without AH and their matched controls.
| Matched healthy controls | Patients with schizophrenia with AH | Patients with schizophrenia without AH | |
|---|---|---|---|
| Sex, F(%)/M(%) | 16(41%)/25(59%) | 16(39%)/25(61%) | 14(40%)/21(60%) |
| Age (range) | 37.43±10.61 (23–68) | 40.02±12.54 years (17–68) | 36.15±9.96 years (18–57) |
| Age when first diagnosed (range) | 21.17±6.04 years (8–32) | 30.88±9.29 years (16–52) | |
| Duration of illness (range) | 15.60±11.78 (2–54) | 6.26±5.87 years (1–20) | |
| Pharmacological treatment* (range) | 503.40±448.74mg/d (66-1850mg/d) | 153.56±89.79mg/d (25–400mg/d) | |
| Duration of the pharmacological treatment | 14.3±6.9 years | UNK |
T1 images were acquired for all participants on 3-Tesla magnets (Achieva, Philips Medical Systems, Best, The Netherlands). A 3D spoiled gradient-echo sequence was used (TE=7.38ms; TR=13.18ms; flip angle=8°, NEX=1, 160 contiguous slices with no interslice gap, acquisition matrix=256×256, FOV=240mm, and voxel size=0.90mm×0.90mm×1mm).
These images were processed for automatic segmentation using FreeSurfer v7.1.129 by default settings. Next, we performed an automatic parcellation of 19 hippocampal subfields and 9 amygdaloid nuclei using the modules designed for these purposes,30,31 which have been built based on a manual delineation combining in vivo and ex vivo data. We next checked whether this automatic segmentation was conducted properly. To do this, we overlapped every hippocampal–amygdaloid segmentation on each normalized MRI for every user using Freeview (incorporated in the FreeSurfer suite) and compared the hippocampal subregions and amygdaloid nuclei to a reference human atlas from the Allen institute.32
In the hippocampus this led to the exclusion of the presubiculum body, and the heads of CA3 and CA1; and in the amygdala we discarded the medial, paralaminar and cortical nuclei. Therefore, we finally considered 16 hippocampal subfields and 6 amygdaloid nuclei per hemisphere. We also extracted the total volume of the hippocampus (THV) and amygdala (TAV), and the estimated total intracranial volume (eTIV) for each subject. A summary of the hippocampal subfields and amygdaloid nuclei analyzed can be found in Supplementary Table 1.
Statistical analysesAll statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 26.0. To make sure the experimental groups were properly matched, we analyzed whether there were any significant differences among groups in the variables age, sex, and eTIV using an analysis of the variance (ANOVA).
To determine whether eTIV and age should be considered covariates for the following volumetric studies, we calculated the Pearson correlation coefficient (ρ) between age, eTIV, and THV for the hippocampus, and TAV for the amygdala. In both correlation analyses, we concluded that only the eTIV should be used as a covariate.
In those cases when the ANCOVA among our three experimental groups was significant, we performed pairwise comparisons and adjusted the significance level using the False Discovery Rate (FDR) method of Benjamini and Hochberg.33 The effect sizes were calculated for all the significant pairwise comparisons (either in relation to the matched controls or to the group of patients with schizophrenia lacking AH). These were calculated using Cohen's d.
To study whether the duration of the illness or the antipsychotic exposure could be influencing the hippocampal and amygdaloid volumes in both cohorts of patients, we performed bivariate correlations among these variables using Pearson's ρ.
To study the relationship between the volume of the affected hippocampal subfields and amygdaloid nuclei in patients with schizophrenia and only the total PSYRATS score, we performed uncorrected bilateral correlations using the Spearman rank-order correlation coefficient (ρ). This could only be performed in those patients that also had the clinical assessment available (26 patients with AH and 25 patients lacking AH).
ResultsDemographicsTo ensure we had properly matched the demographic traits of our three experimental groups, we compared the age and eTIV among them. There were no significant differences regarding their age (F2,126=1.237, p=0.294) or eTIV (F2,126=1.261, p=0.287).
Determination of covariatesNext, to determine the possible covariates for the following volumetric analyses, we performed two correlation analyses to see whether age or eTIV would influence the THV or TAV. In the hippocampus, age was not correlated with the THV (ρ=−0.135, p=0.063) whereas the eTIV was significantly correlated (ρ=0.200, p=0.011). The amygdala analysis rendered similar results: while age was not correlated (ρ=−0.125, p=0.079), eTIV was significantly correlated with TAV (ρ=0.505, p=5.215×10−10). Therefore, in both cases we only considered eTIV as a covariate for the consequent volumetric analyses.
Influence of the duration of the illness and antipsychotic exposure on total volume of the amygdala and hippocampusTo make sure the duration of the illness or the antipsychotic exposure were not influencing the volumetric differences we see in AH and NAH groups, we then proceeded to perform correlations between these two variables, and the THV and TAV. We did not find any significant relationships. In the hippocampus, the illness duration was not correlated with its volume (AH: ρ=−0.100, p=0.620; NAH: ρ=−0.147, p-value=0.474), and similar results were found in the amygdala (AH: ρ=−0.235, p=0.238; NAH: ρ=−0.159, p=0.474). Regarding the antipsychotic exposure there were no significant correlations in the amygdala (AH: ρ=−0.424, p=0.062; NAH: ρ=0.115, p=0.569) or the hippocampus (AH: ρ=−0.424, p=0.062; NAH: ρ=0.077; p-value=0.703).
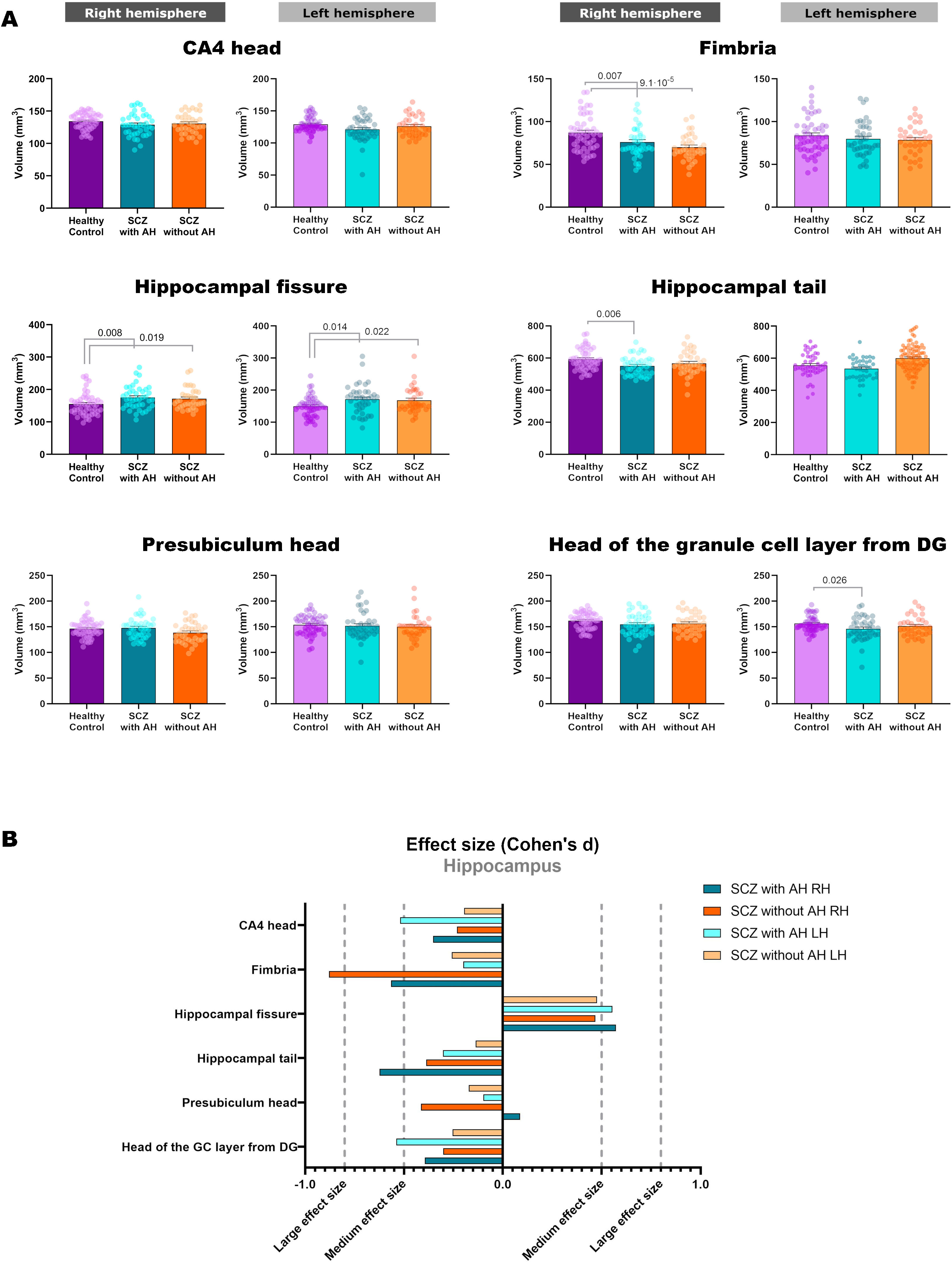
Volumetric analysis of the hippocampal subfields in patients with schizophrenia with and without auditory hallucinationsWe then performed volumetric analyses of the whole hippocampus and its different subfields (Fig. 1 and Table 2). Despite not finding significant alterations in the whole hippocampus from the right (RH: p=0.123) and left hemispheres (LH: p=0.211), we detected interesting variations in different hippocampal regions. There were effect trends in the volume of the right presubiculum head (RH: p=0.094) and the head of the left CA4 (LH: p=0.053).
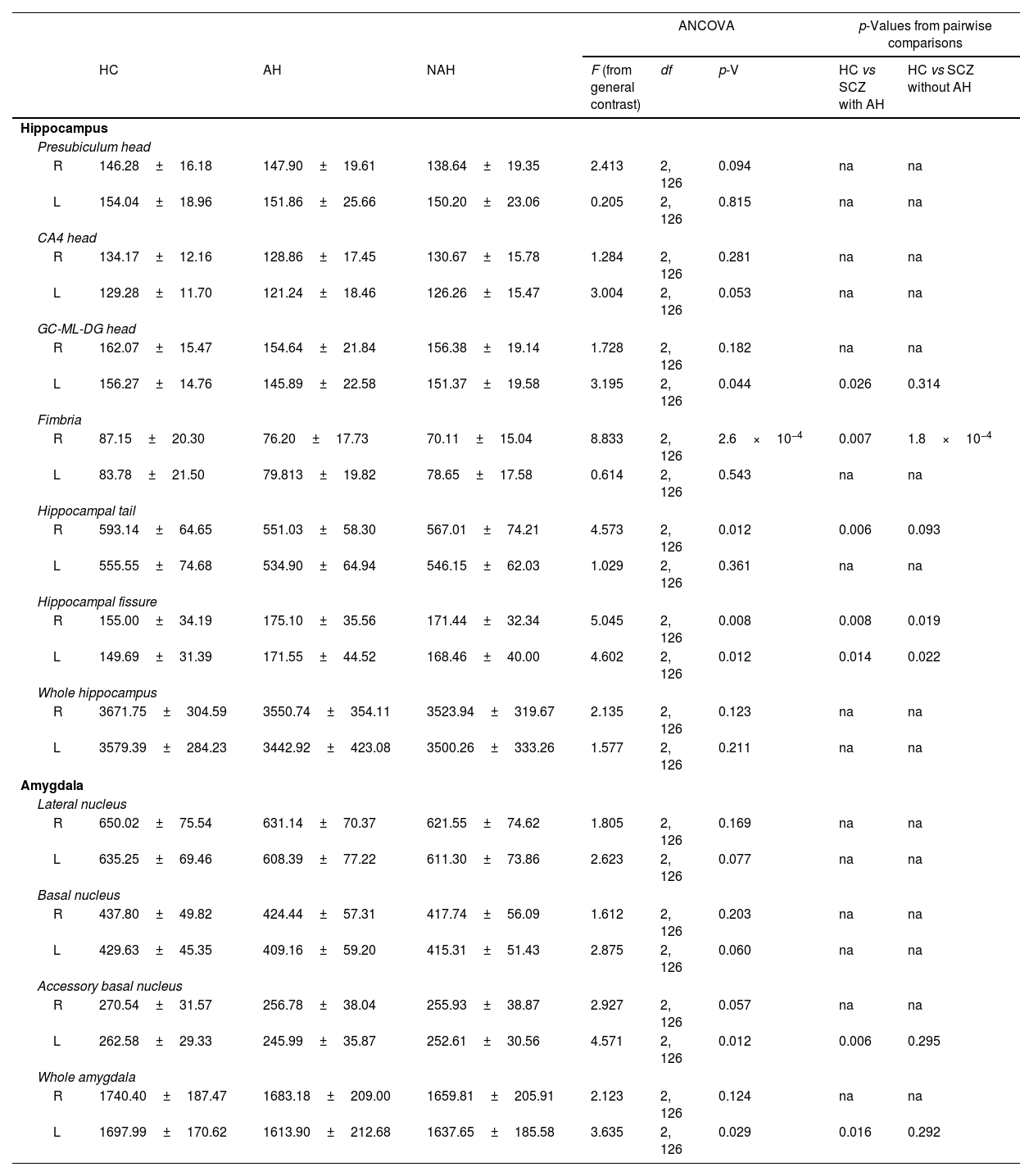
Statistical results from the hippocampus and amygdala segmentation (only significant nuclei and contralateral equivalent).
| ANCOVA | p-Values from pairwise comparisons | |||||||
|---|---|---|---|---|---|---|---|---|
| HC | AH | NAH | F (from general contrast) | df | p-V | HC vs SCZ with AH | HC vs SCZ without AH | |
| Hippocampus | ||||||||
| Presubiculum head | ||||||||
| R | 146.28±16.18 | 147.90±19.61 | 138.64±19.35 | 2.413 | 2, 126 | 0.094 | na | na |
| L | 154.04±18.96 | 151.86±25.66 | 150.20±23.06 | 0.205 | 2, 126 | 0.815 | na | na |
| CA4 head | ||||||||
| R | 134.17±12.16 | 128.86±17.45 | 130.67±15.78 | 1.284 | 2, 126 | 0.281 | na | na |
| L | 129.28±11.70 | 121.24±18.46 | 126.26±15.47 | 3.004 | 2, 126 | 0.053 | na | na |
| GC-ML-DG head | ||||||||
| R | 162.07±15.47 | 154.64±21.84 | 156.38±19.14 | 1.728 | 2, 126 | 0.182 | na | na |
| L | 156.27±14.76 | 145.89±22.58 | 151.37±19.58 | 3.195 | 2, 126 | 0.044 | 0.026 | 0.314 |
| Fimbria | ||||||||
| R | 87.15±20.30 | 76.20±17.73 | 70.11±15.04 | 8.833 | 2, 126 | 2.6×10−4 | 0.007 | 1.8×10−4 |
| L | 83.78±21.50 | 79.813±19.82 | 78.65±17.58 | 0.614 | 2, 126 | 0.543 | na | na |
| Hippocampal tail | ||||||||
| R | 593.14±64.65 | 551.03±58.30 | 567.01±74.21 | 4.573 | 2, 126 | 0.012 | 0.006 | 0.093 |
| L | 555.55±74.68 | 534.90±64.94 | 546.15±62.03 | 1.029 | 2, 126 | 0.361 | na | na |
| Hippocampal fissure | ||||||||
| R | 155.00±34.19 | 175.10±35.56 | 171.44±32.34 | 5.045 | 2, 126 | 0.008 | 0.008 | 0.019 |
| L | 149.69±31.39 | 171.55±44.52 | 168.46±40.00 | 4.602 | 2, 126 | 0.012 | 0.014 | 0.022 |
| Whole hippocampus | ||||||||
| R | 3671.75±304.59 | 3550.74±354.11 | 3523.94±319.67 | 2.135 | 2, 126 | 0.123 | na | na |
| L | 3579.39±284.23 | 3442.92±423.08 | 3500.26±333.26 | 1.577 | 2, 126 | 0.211 | na | na |
| Amygdala | ||||||||
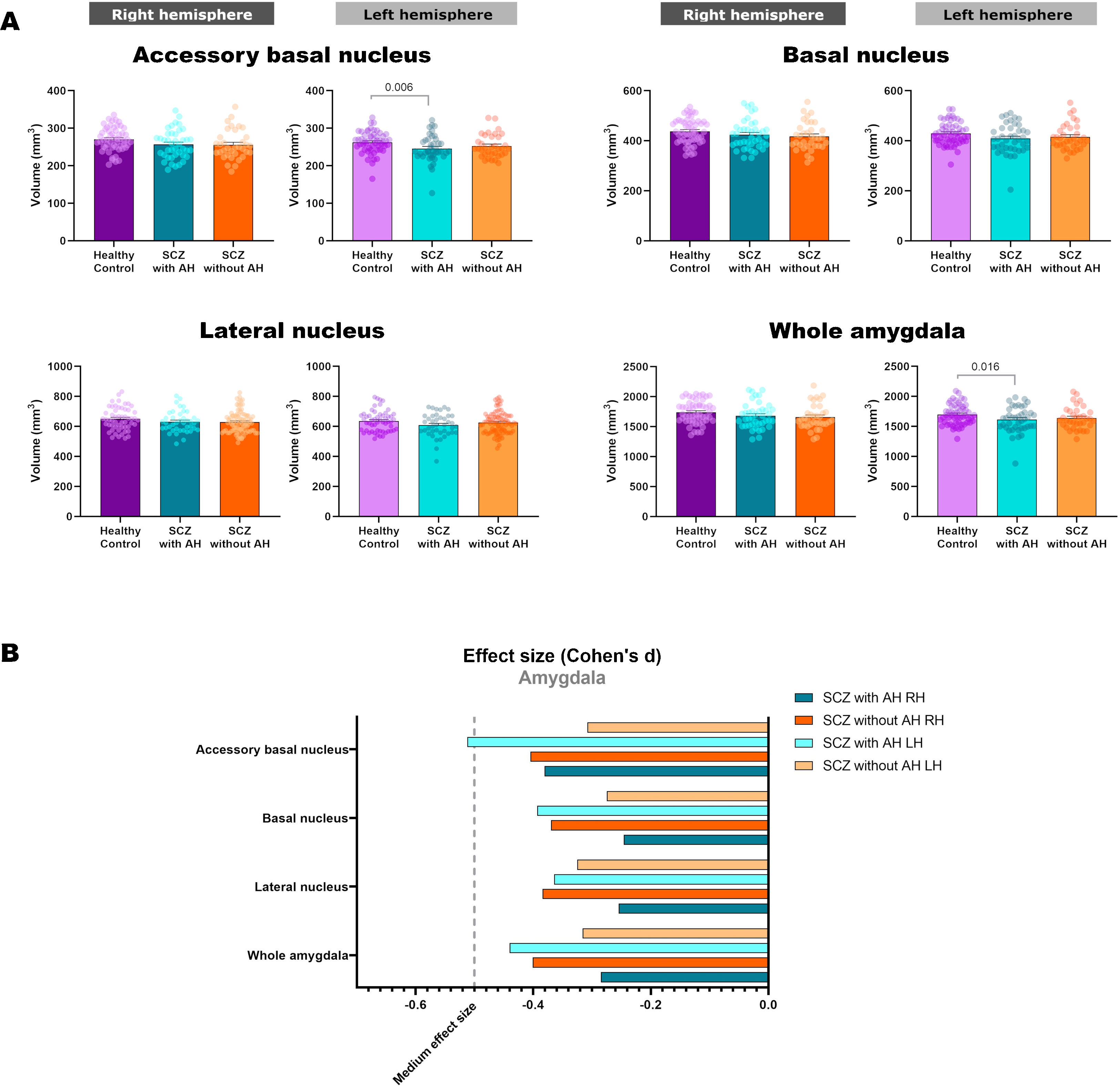
| Lateral nucleus | ||||||||
| R | 650.02±75.54 | 631.14±70.37 | 621.55±74.62 | 1.805 | 2, 126 | 0.169 | na | na |
| L | 635.25±69.46 | 608.39±77.22 | 611.30±73.86 | 2.623 | 2, 126 | 0.077 | na | na |
| Basal nucleus | ||||||||
| R | 437.80±49.82 | 424.44±57.31 | 417.74±56.09 | 1.612 | 2, 126 | 0.203 | na | na |
| L | 429.63±45.35 | 409.16±59.20 | 415.31±51.43 | 2.875 | 2, 126 | 0.060 | na | na |
| Accessory basal nucleus | ||||||||
| R | 270.54±31.57 | 256.78±38.04 | 255.93±38.87 | 2.927 | 2, 126 | 0.057 | na | na |
| L | 262.58±29.33 | 245.99±35.87 | 252.61±30.56 | 4.571 | 2, 126 | 0.012 | 0.006 | 0.295 |
| Whole amygdala | ||||||||
| R | 1740.40±187.47 | 1683.18±209.00 | 1659.81±205.91 | 2.123 | 2, 126 | 0.124 | na | na |
| L | 1697.99±170.62 | 1613.90±212.68 | 1637.65±185.58 | 3.635 | 2, 126 | 0.029 | 0.016 | 0.292 |
In addition, the left head of the granule cell layer of the DG showed a significant volume decrease (LH: p=0.044), which could only be detected in patients with AH (LH: p=0.026), with a moderate effect size (LH: d=−0.539). There were also volume reductions in the right fimbria (RH: p=2.6×10−4), when comparing each group of patients with healthy controls. These reductions were significant when comparing patients with schizophrenia and AH (RH: p=0.007), displaying a moderate effect size (RH: d=−0.565), and when comparing patients with schizophrenia without AH (RH: p=1.8×10−4), which displayed a large effect size (RH: d=−0.879). The right hippocampal tail was also affected by the disease (RH: p=0.012) and this effect was due to a reduction in its volume only in patients with schizophrenia and AH (RH: p=0.006), showing a moderate effect size (RH: d=−0.624). Last of all, there was a significant bilateral volume increase of the hippocampal fissure (RH: p=0.008; LH: p=0.012). Pairwise comparisons revealed that both patients with and without AH had larger hippocampal fissures than matched healthy controls. This was significant in the right and left fissures of patients with AH (RH: p=0.008; d=0.573; LH: p=0.014; d=0.554) and without AH (RH: p=0.019, d=0.468; LH: p=0.022, d=0.476).
Volumetric analysis of the amygdaloid nuclei in patients with schizophrenia with and without auditory hallucinationsNext, we aimed to study whether schizophrenia and AH affected the volume of the whole amygdala and different amygdaloid nuclei (Fig. 2 and Table 2). Regarding its total volume, we found a significant effect in the amygdala from the left hemisphere (LH: p=0.029), with significant reductions only in patients with AH (p=0.016), which displayed small effect sizes (d=−0.440).
When studying the volume of the different amygdaloid nuclei, we found effect trends in the volumes of the left lateral nucleus (LH: p=0.077) and the left basal nucleus (LH: p=0.060). Regarding the accessory basal nucleus, there was a significant reduction of its volume in the left hemisphere (LH: p=0.012) and a trend toward a decrease in the right hemisphere (LH: p=0.057). This effect was due to a reduction of the volume of the left nucleus only in patients of schizophrenia with AH (LH: p=0.006), which displayed a moderate effect size (d=−0.512).
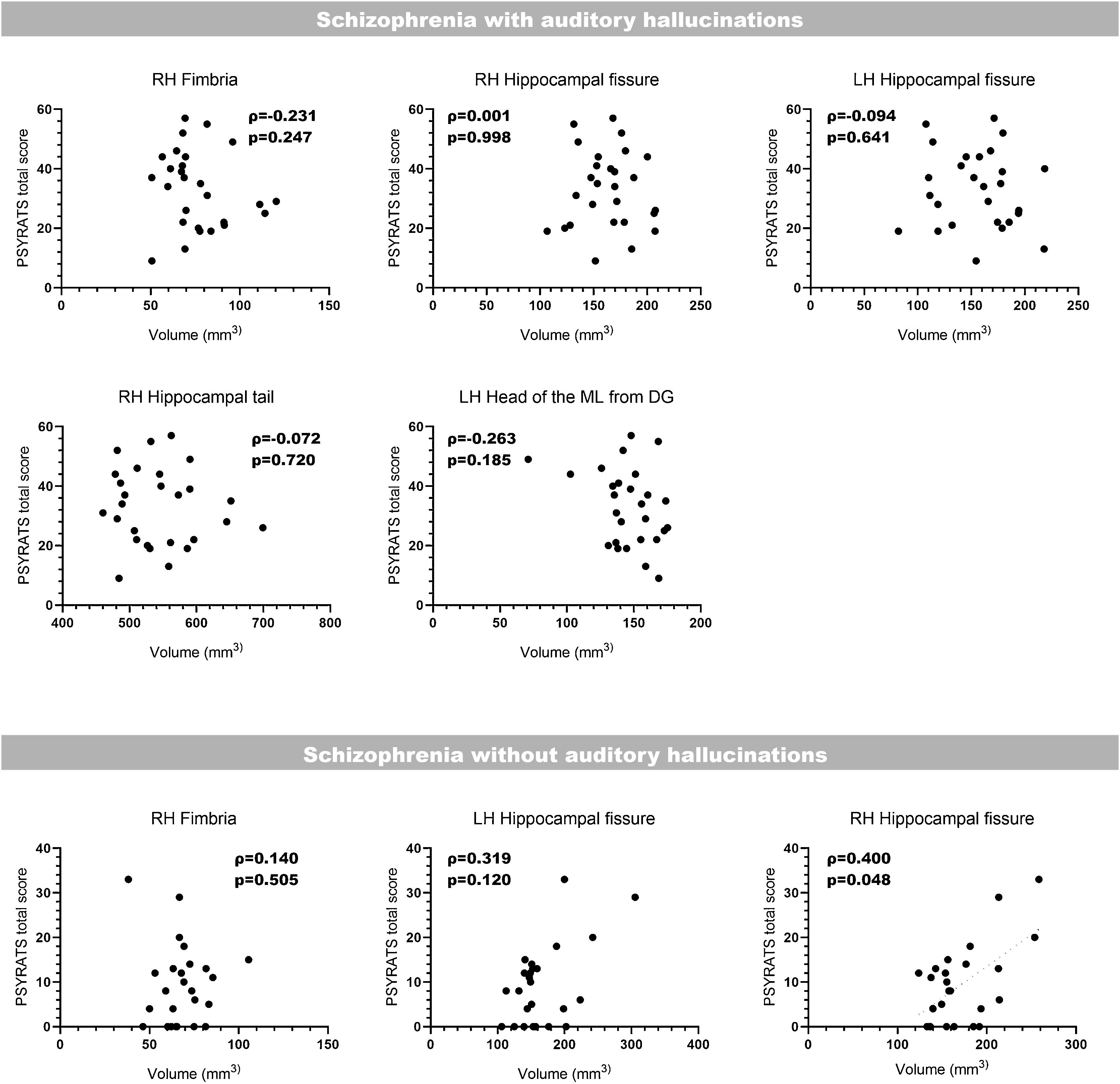
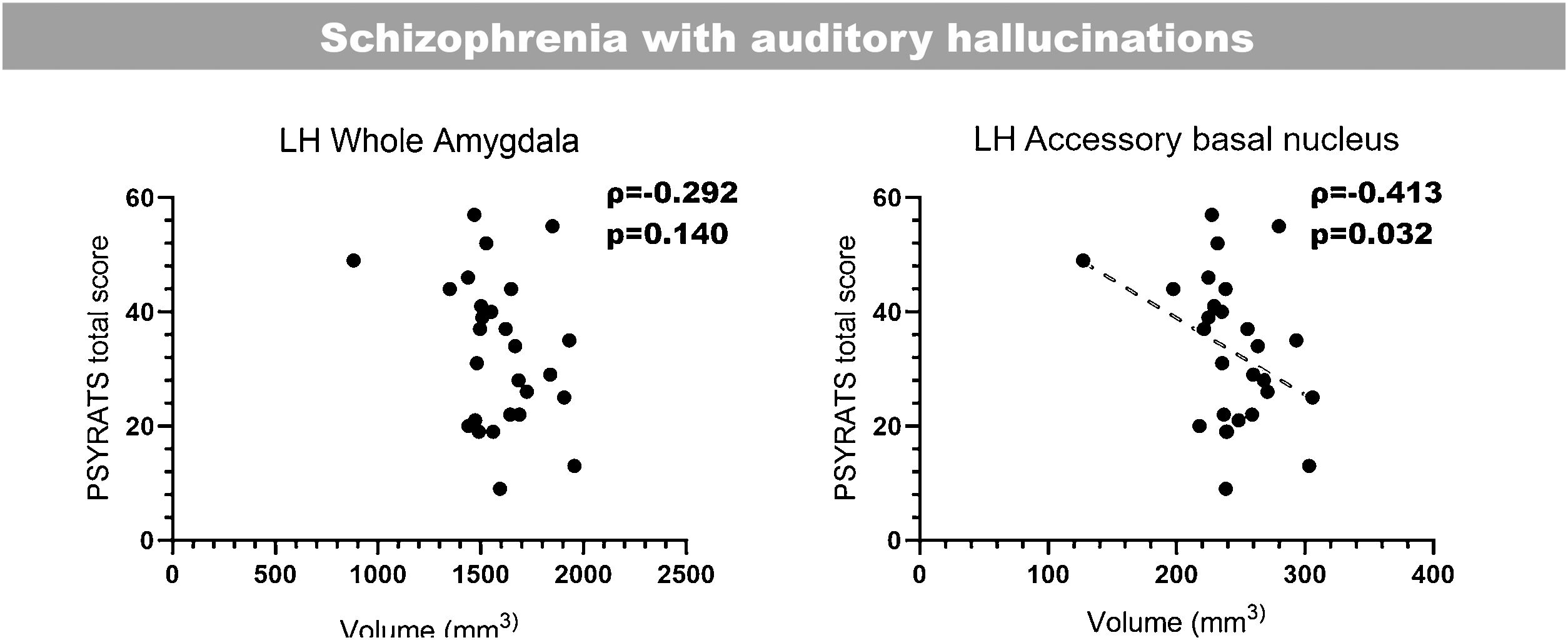
Correlation with clinical scoresLast of all, we performed bilateral correlations between the total PSYRATS score and the volumes of the affected hippocampal subfields (Fig. 3) and amygdaloid nuclei (Fig. 4), in patients with schizophrenia with and without AH. In the hippocampus, only patients without AH showed a significant positive correlation between the volume of the right fimbria and the total PSYRATS score (ρ=0.400, p=0.048). In the amygdala we found a significant negative correlation between the volume of its left accessory basal nucleus and the total PSYRATS score in patients with schizophrenia and persistent AH (ρ=−0.413, p=0.032).
Bilateral correlations between the total PSYRATS score and the volumes of those hippocampal subfields that were affected in patients with schizophrenia with and without auditory hallucinations. These correlations were performed only between the PSYRATS score and the affected hippocampal subfields, and were not corrected (Spearman's rank-order coefficient).
Bilateral correlations between the total PSYRATS score and the volumes of those amygdaloid nuclei that were affected in patients with schizophrenia with and without auditory hallucinations (Spearman's rank-order coefficient). These correlations were performed only between the PSYRATS score and the affected amygdaloid nuclei, and were not corrected (Spearman's rank-order coefficient).
In this study, we have shown volumetric variations of different hippocampal subfields and amygdaloid nuclei analyzing separate cohorts of chronic patients with schizophrenia and AH, and patients with schizophrenia without AH.
Regarding the hippocampus, we have found alterations in several subfields, although the total hippocampal volume remained unchanged. Decreases in the volume of the whole hippocampal formation have been one of the classical hallmarks of schizophrenia when analyzing MRIs.34 However, it is known that the type of antipsychotic treatment received by the patients may interfere with this volume loss reported in the whole hippocampus.18 This could explain why we have not found differences in our sample, which has not been controlled for this trait. Despite not finding this expected volumetric reduction, we did find a few interesting volumetric changes in different hippocampal subfields. We report a significant volume loss of the right fimbria in patients with and without AH. This is in line with several reports that have described alterations related to myelin and oligodendrocytes in postmortem and neuroimaging studies of patients with schizophrenia.35 Specifically, previous reports have already shown that patients with schizophrenia show reduced fractional anisotropy and/or increased radial diffusivity, two measures of decreased white matter integrity, in the fornix,36,37 which was more pronounced in the right hemisphere, at least in first episode patients.38 Because the hippocampal fimbria expands ventrally to become the fornix,4 it is not surprising that we report similar decreases.
On the other hand, we have also shown a bilateral increase in the volume of the hippocampal fissure both in patients with and without persistent AH. These results, along with the volumetric reductions of the fimbria, are partially in line with those reported by Tesli et al. (2020), which show a reduced volume of the fimbria and a bigger hippocampal fissure, among other changes, in patients of schizophrenia with a history of violence.17 We did not register this trait in our cohorts of patients, but it would be interesting to have this data in future recruitments to determine if the volumetric variations were due to the presence of AH or other demographic traits. The hippocampal fissure is an expansion of the lateral ventricles,4 consequently, the increase in the volume of this structure must be connected to the enlargement of these ventricles, which is one of most robust structural findings in schizophrenia.39
Interestingly, in our study we show that only patients that experience AH have decreases in the right hippocampal tail. These reductions are completely in agreement with previous results showing a decrease in the right and left hippocampal tails in patients with schizophrenia and persistent AH,22 as well as in chronic patients of this disorder.15 In addition, we have also shown reductions of the left head of the granule cell layer of the DG only in patients with persistent AH. This result coincides with previous MRI reports,15,40 and a postmortem volumetric study41 performed in a general cohort of patients. Furthermore, reductions in the number of granule cells have also been described in the hippocampus of patients.42 Interestingly, a recent report proposes that the incorrect maturity of this layer may partially be responsible for the onset of this disease.16 Our results suggest that these alterations may be more pronounced, or even exclusive, of patients with AH. In fact, there is evidence from studies in humans and animal models of the possible involvement of granule cell generation and development, both during development and adulthood, in the etiopathology of schizophrenia.43
On the other hand, we have also described significant volumetric reductions of the left amygdala and its accessory basal nucleus. Interestingly, these alterations were exclusive to the cohort of patients that experienced persistent AH. In agreement with our present results, previous studies focusing on patients with AH have also found significant volume reductions of the left amygdala,24 and a previous report from our research group also found a decrease of its gray matter in chronic patients with AH.23 It has been hypothesized that these volumetric reductions may be related to the emotional effect of the persistent AH. In fact, functional neuroimaging studies have found increased activity of the amygdala in response to emotional words resembling those heard by the patients.44,45
Likewise, we show that the volume of the left accessory basal nucleus was decreased only in patients with persistent AH. These results are in line with the volume shrinkage reported in this nucleus in a general group on schizophrenia patients,20 or in patients with schizophrenia and a history of violence.17 Interestingly, we also found reductions in the volume of this nucleus in a mouse model of the disease46 and postmortem studies have also revealed structural and neurochemical alterations.47,48 Although the function of the accessory basal or basomedial nucleus has not been studied in detail, it is interesting to note that it participates actively, together with the basolateral nucleus, in the fear response.49 More relevant to our findings in patients with AH, the accessory basal nucleus mediates the effect of auditory stimuli coming from the medial geniculate nucleus through the lateral amygdaloid nucleus and projects to the central amygdala to initiate fear responses.50 It is tempting to hypothesize that the prolonged exposure to AH, which are frequently distressing, ultimately may have modified this circuit involving the accessory basal nucleus. In fact, in a recent report we have described important volumetric reductions in the medial geniculate nucleus of patients with schizophrenia and AH.27
Interestingly, and in connection with the possible involvement of the accessory basal nucleus in the response to AH, we have also observed a significant negative correlation between its volume in patients suffering AH and the total PSYRATS score.25 These scales have already been very useful to characterize different traits of AH in clinical research, including its correlation with gray matter concentrations of patients with persistent AH,23,26 and more recently, with the volume of several thalamic nuclei in the very same cohort of patients used in this report.27
Altogether, we describe decreases in the volume of several limbic regions and nuclei only present in patients of schizophrenia that also show persistent AH, and alterations in regions that are also present in those patients lacking AH. Therefore, the alterations of the hippocampal fimbria and fissure may be caused by factors that were not accounted for in our study, while the decreases in the volumes of the hippocampal tail, part of the dentate gyrus, as well as the whole amygdala and its accessory basal nucleus, may be caused by the presence of these hallucinations. In addition, the illness duration and antipsychotic exposure might be important to explain part of our results. In fact, the latter has been reported to decrease the brain volume51,52 and might be influencing differentially schizophrenia patients with and without AH. However, we did not find that these traits exerted any influence in the total volumes of the amygdala and hippocampus in our study. Replicative studies with larger numbers of patients should be performed to exclude the influence of these factors.
Further research should address the possibility of a history of violence in these patients, to discern this confounding variable, as well as a more detailed assessment of the AH. However, with our report we provide important information on different regions of these limbic structures, which can contribute to a better understanding of schizophrenia with persistent AH and can set the basis for providing a better tool to facilitate the diagnosis of this disorder.
FundingThis work was supported by the projects RTI2018-098269-B-I00 and PID2021-127595OB-100 financed by the Spanish Ministry of Science and Innovation/AEI/10.13039/501100011033/ (“FEDER Una manera de hacer Europa”), the Generalitat Valenciana (PROMETEU/2020/024), Spanish Ministry of Health: FIS PI20/00473, La Marató de TV3 (grants number 091230 and 091231) and partly funded by FEDER funds of the EU and CERCA Programme (Generalitat de Catalunya). MP-R was supported by an “Atracció de Talent” grant from the University of Valencia.
Conflict of interestNone.