Clozapine is an effective antipsychotic. However, its use has been associated with agranulocytosis. For this reason, it has been restricted for the treatment of resistant schizophrenia under a strict haematologic control. The objective of this work was to assess the risk of haematologic dyscrasias in a sample of clozapine-treated patients in a 5-year period.
Materials and methodThis is a follow-up study in a cohort of clozapine-treated patients in which the risk of haematological dyscrasias was assessed. Complete blood cell count was made for each patient in a weekly basis for the first 18 weeks and thereafter monthly.
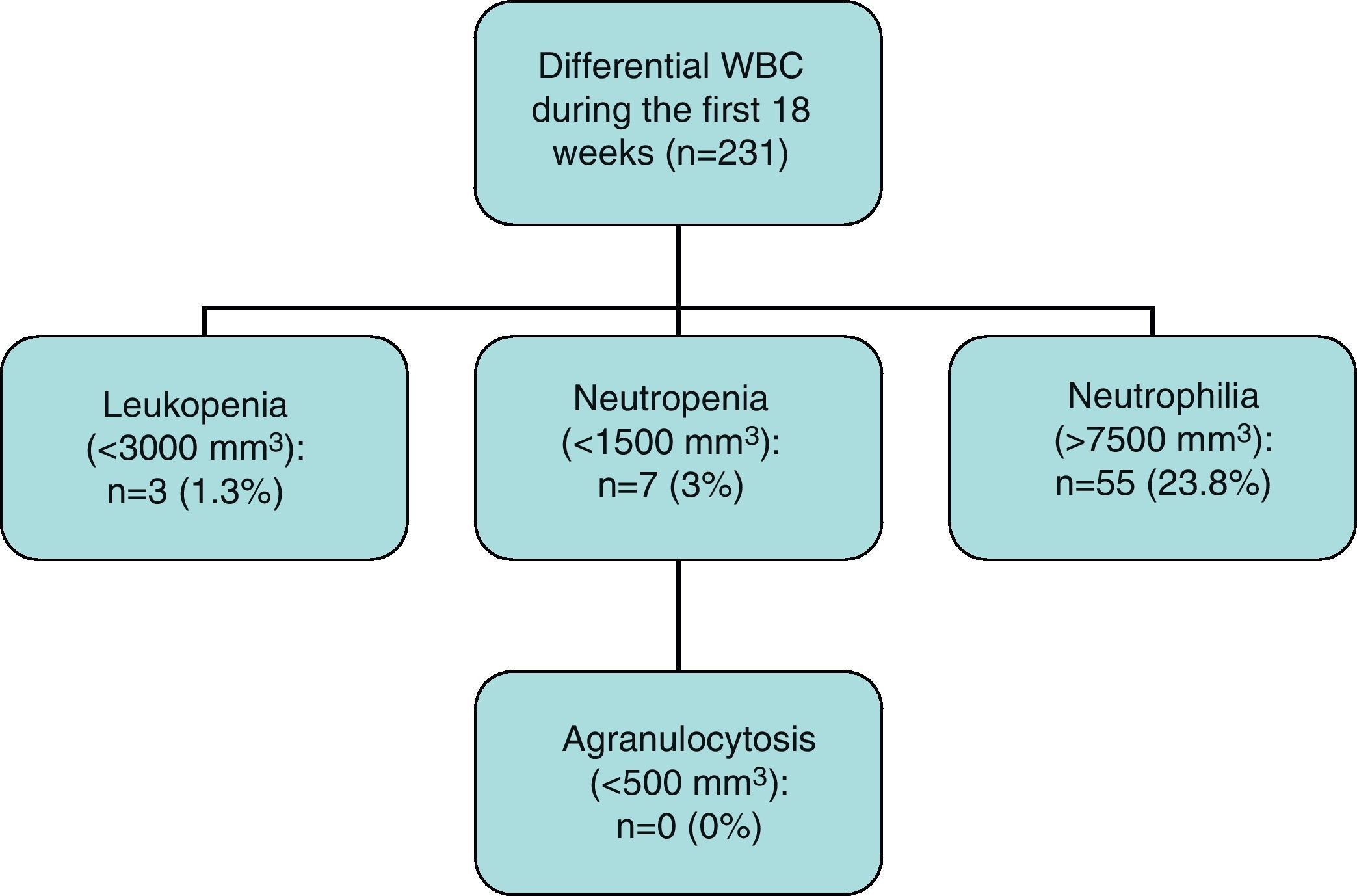
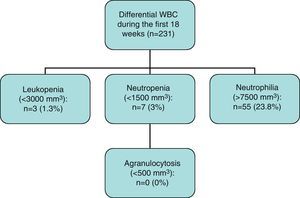
Results271 patients in treatment with clozapine were followed up. The mean age was 32.3 years, with 36.5% women. The mean dose was 2276mg, ranging from 25 to 600mg/day. During the first 18 weeks of follow-up, we observed a 3% incidence of neutropenia and 1.3% of leucopenia. During the next 2 years, only 1 new case of neutropenia and leucopenia was observed (n=120). No new cases were observed during the rest of follow up (n=69). No cases of agranulocytosis were observed.
ConclusionsA 3% incidence of neutropenia concentrated in the first months of follow up and no cases of agranulocytosis were observed in our sample. Actual evidence on clozapine effectiveness and safety and the results of this study suggests that a critical revision of follow-up protocols is suitable.
La clozapina es un fármaco de probada efectividad, sin embargo, su uso se asoció a la aparición de agranulocitosis y así, fue restringido para esquizofrenia resistente y con un estricto control hematológico. El objetivo de nuestro trabajo es evaluar el riesgo de discrasias sanguíneas en una población de pacientes en tratamiento con clozapina seguidos durante 5 años.
Material y métodosSe trata de una cohorte de pacientes en tratamiento con clozapina en los que se evaluó el riesgo de aparición de alteraciones en la serie blanca, a través de analíticas semanales durante las primeras 18 semanas y mensuales a partir de entonces.
ResultadosSe trata de una muestra de 271 pacientes, con un media de edad de 32, 3 años y un 63,5% de hombres. La dosis media administrada fue de 227,6mg/día (25 a 600mg). Durante las primeras 18 semanas de seguimiento, se observó una incidencia de neutropenia de 3,0% y de leucopenia de 1,3%. Durante los dos años siguientes, se encontró un nuevo caso de leucopenia y uno de neutropenia (n=120). No se observaron nuevos casos en los pacientes que completaron 5 años de seguimiento (n=69). A lo largo de todo el seguimiento, no hubo ningún caso de agranulocitosis.
ConclusiónSe observó una incidencia de neutropenia del 3%, concentrado en los primeros meses de seguimiento. Ningún paciente presentó agranulocitosis. La evidencia actual disponible sobre efectividad y seguridad de la clozapina y los resultados del presente estudio, hacen adecuado plantearse revisar los protocolos de seguimiento y utilización de este fármaco.
Clozapine, a second generation antipsychotic, is the treatment of choice for patients with refractory schizophrenia, as it has proved to be more effective than other existing antipsychotics, both typical and atypical.1–7 Discovered in 1958, it was the first atypical antipsychotic, effective in the treatment of positive and negative schizophrenic symptoms,8,9 which in addition to its favourable profile regarding extrapyramidal adverse effects, generated grand expectations in the scientific and clinical world at that time. However, its development and commercialisation were interrupted in 1975, due to information concerning patients who developed agranulocytosis in Finland while being treated with clozapine.8,10,11
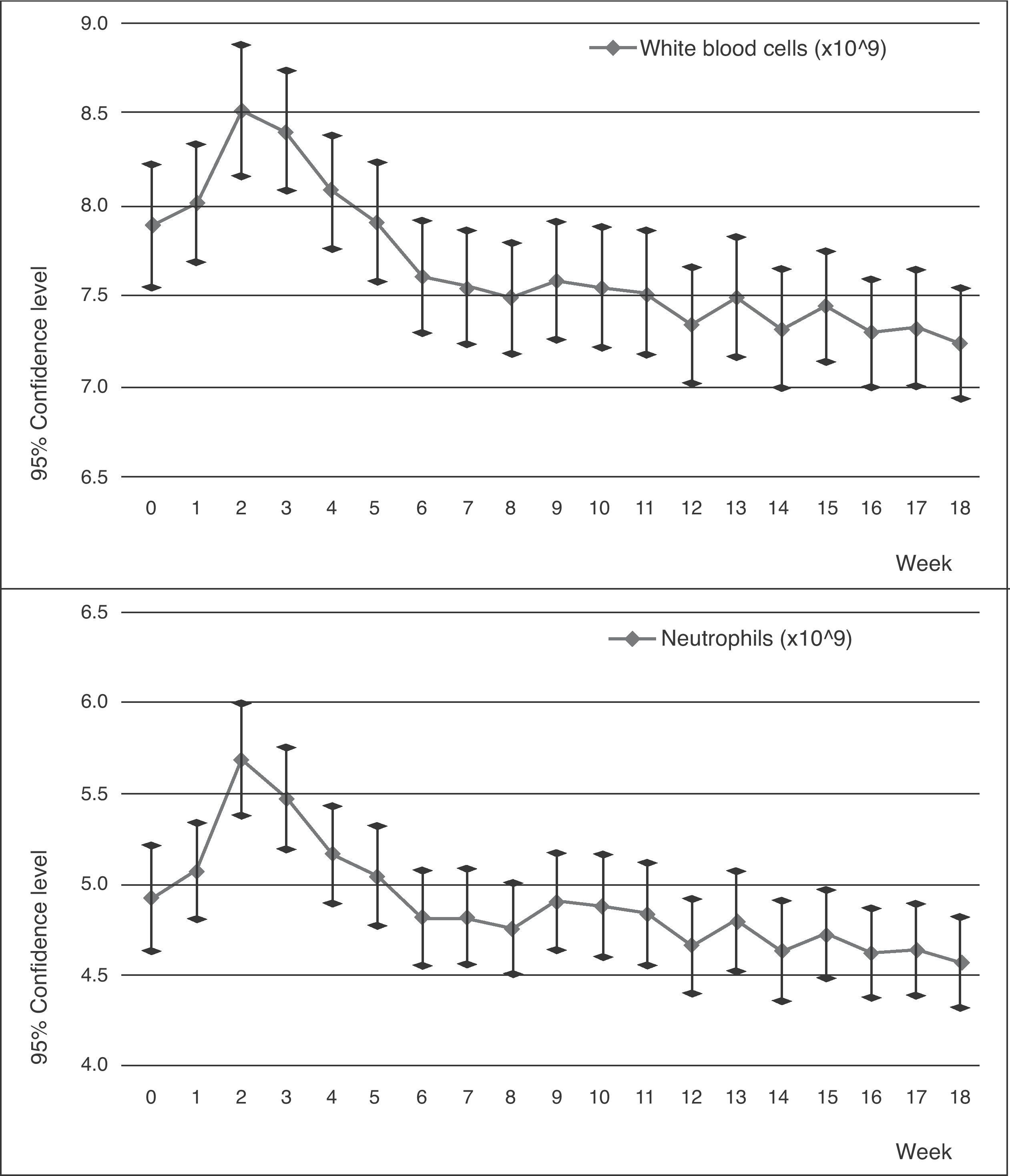
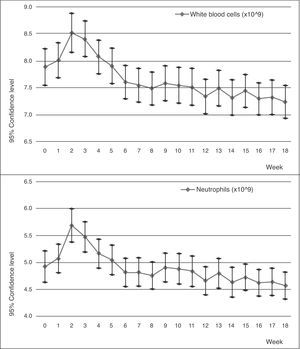
In later years, studies involving blood monitoring were carried out that showed clinically favourable results.7,12 The use of clozapine was approved by the Food and Drug Administration (FDA) in 1990 as an exclusive treatment for patients with resistant schizophrenia, who would also be subject to strict blood monitoring. Since then, clozapine was progressively reintroduced in 1993 under strict conditions put forth by the Ministry of Health, who insured its controlled use by limiting its applications and enforcing a strict check of differential white blood cell counts before starting treatment, on a weekly basis for the first 18 weeks and on a monthly basis after that (Figs. 1 and 2).
The incidence of agranulocytosis and neutropenia show marked variations, depending on the sample size and the location in which the study was completed.13 In the follow-up cohorts with a larger sample size, an incidence of approximately 1% and 3% was observed for agranulocytosis and neutropenia respectively.14–16 Of the agranulocytosis cases, 95% appeared within the first 6 months of treatment, with the risk being even greater in the first 3 months.16 The exact mechanism by which clozapine induces agranulocytosis is not completely clear. There is evidence that it involves an immune-mediated reaction17 and that certain factors like ethnicity and age of the populations studied could intervene.14–16,18–21 We realised how important this drug is for treating resistant patients and how little data regarding the risk of agranulocytosis there is in our population. Consequently, in an effort to evaluate the risk of developing blood dyscrasias, we prepared this prospective 5-year follow-up of a cohort of patients undergoing treatment in the Clozapine Clinic (Tables 1 and 2).
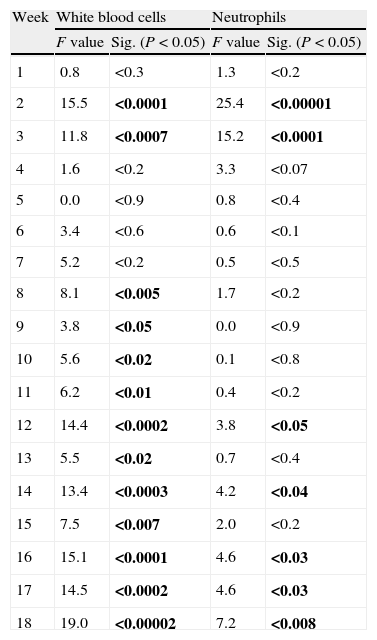
Repeated measures analysis of variance. Within-subject contrasts were also performed a priori comparing each measure with the initial one. F and P values (in bold, P<0.05), according to Pillai's Trace, for the white blood cell and neutrophil count during the first 18 weeks of follow-up.
| Week | White blood cells | Neutrophils | ||
| F value | Sig. (P<0.05) | F value | Sig. (P<0.05) | |
| 1 | 0.8 | <0.3 | 1.3 | <0.2 |
| 2 | 15.5 | <0.0001 | 25.4 | <0.00001 |
| 3 | 11.8 | <0.0007 | 15.2 | <0.0001 |
| 4 | 1.6 | <0.2 | 3.3 | <0.07 |
| 5 | 0.0 | <0.9 | 0.8 | <0.4 |
| 6 | 3.4 | <0.6 | 0.6 | <0.1 |
| 7 | 5.2 | <0.2 | 0.5 | <0.5 |
| 8 | 8.1 | <0.005 | 1.7 | <0.2 |
| 9 | 3.8 | <0.05 | 0.0 | <0.9 |
| 10 | 5.6 | <0.02 | 0.1 | <0.8 |
| 11 | 6.2 | <0.01 | 0.4 | <0.2 |
| 12 | 14.4 | <0.0002 | 3.8 | <0.05 |
| 13 | 5.5 | <0.02 | 0.7 | <0.4 |
| 14 | 13.4 | <0.0003 | 4.2 | <0.04 |
| 15 | 7.5 | <0.007 | 2.0 | <0.2 |
| 16 | 15.1 | <0.0001 | 4.6 | <0.03 |
| 17 | 14.5 | <0.0002 | 4.6 | <0.03 |
| 18 | 19.0 | <0.00002 | 7.2 | <0.008 |
The study involves a cohort of patients treated in the Clozapine Clinic of Outpatient Care at the Neuroscience Institute in the Hospital Clínico in Barcelona. Patients fulfilled the criteria defined by the ICD-10 for diagnosis of schizophrenia, schizoaffective disorder or bipolar disorder with psychotic symptoms. They began treatment with clozapine due to their resistance to previous antipsychotic treatment. This resistance is defined by the persistence of psychotic symptoms despite consecutive treatment with therapeutic dosages of 2 antipsychotics, administered within 6 months of each other; or also by intolerance to treatment with other antipsychotics due to serious adverse or untreatable neurological reactions (extrapyramidal symptoms or tardive dyskinesia).
For these patients, a white blood cell count and absolute neutrophil count were performed, according to current regulations, weekly during the first 18 weeks of treatment and monthly subsequently.
The plasma concentrations of clozapine and norclozapine were determined by high performance liquid chromatography in a subgroup of 112 patients. We included all of the patients who joined the study after the technique was available in our laboratory in this subgroup.
With reference to the blood evaluations, we considered as criteria for inclusion that the cases had had at least 4 months of follow-up in our centre, and no less than 24 months follow-up for those cases from another centre. The patients who began clozapine treatment before the regulations that dictate follow-up procedure went into effect were also included.22
For the purpose of this study, the definitions used for leucopenia, neutropenia and agranulocytosis are the internationally accepted ones. Leucopenia was defined by a white blood cell count of less than 3×109/L and neutropenia was a neutrophil count between 0.5×109/L and 1.5×109/L. Agranulocytosis was defined as a granulocyte count of less than 0.5×109/L. Likewise, the definitions of neutrophilia and leucocytosis were a neutrophil count of less than 7.5×109/L and a white blood cell count less than 11.5×109/L respectively. In this study, the time criterion was defined as 3 consecutive abnormal counts during the weekly follow-up and 2 during the monthly follow-up.
For the analysis of the differences in neutrophil and white blood cell values in the determinations performed, from the initial measure to the following measures, variance analysis was used for repeated measures. To determine the points where significant differences occurred, contrasts that were a priori within subjects were calculated, comparing each determination with the initial one.
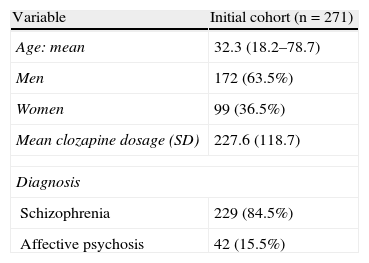
ResultsThe sample consisted of 271 patients, 63.5% of whom were male. The mean age was 32.3 years (standard deviation [SD] 10.5), with an interval that went from 18.2 to 78.7 years. The dosages administered of clozapine during the follow-up were between 25 and 600mg/day. The mean dose administered was 227.6mg/day (SD 118.7), and the median was 200mg/day.
The plasma concentrations of clozapine and norclozapine were monitored in a subgroup of 112 patients from the cohort, composed of 38 females with a mean dose of 207.6mg/day and 74 males with a mean dose of 256mg/day. The plasma concentrations of clozapine and norclozapine were 239.1ng/ml and 138.2ng/ml respectively for women, and 274.7ng/ml and 166.3ng/ml respectively for men.
Of the total 231 cases that completed the first 18 weeks of follow-up, 3 cases of leucopenia (1.3%), 7 cases of neutropenia (3.0%) and no cases of agranulocytosis were observed.
The sharp increase in the neutrophil and white blood cell numbers during the first few weeks attracted attention. This increase reached its maximum point between the second and third week, with there being a significant difference between these counts and the base count. After this point, the count began to decrease progressively until the difference became significant during the last 4 weeks for the neutrophils and during the last 10 weeks for the white blood cells.
During the next 2 years of follow-up, this difference maintained significance (P<0.005 according to Pillai's Trace), although the counts tended to stabilise. One new case of leucopenia and neutropenia was observed of the total sample (n=120).
We had a complete monthly follow-up of haematological counts during 5 years for 69 patients. During these years, no new cases of leucopenia or neutropenia were observed and the curve of the count tended to level out towards the limits.
Of the 2 cases mentioned, we decided to suspend treatment in 1 of them in an effort to keep neutrophil numbers low and, in the other, due to the emergence of simple epileptic seizures.
DiscussionIn the cohort of patients treated with clozapine, a neutropenia prevalence of 3% was observed, concentrated in the first few months of follow-up, which agrees with data published by other authors.23 No patient presented agranulocytosis.
The majority of patients in the sample had a diagnosis of resistant psychosis or had presented significant adverse effects associated with the use of other antipsychotic treatments.
Estimations on resistant schizophrenia prevalence vary depending on its definition, but it is estimated that between one-fifth and one-third of patients with schizophrenia have a sub-optimal response to antipsychotic treatment. This warrants attention in that, despite current scientific evidence for the benefits of clozapine over other antipsychotics in schizophrenia treatment, this drug continues to be underused.24 In the United States, the percentage of clozapine prescriptions compared to other antipsychotics did not reach 3.5% during the 2008–2009 period.25 In an Italian sample, the percentage observed was 1.5%.26
The prescription of clozapine seems to be limited by its adverse effects like drooling, dizziness, constipation and other symptoms that could potentially increase the morbidity and mortality rate among patients, such as metabolic syndrome, agranulocytosis, myocarditis, cardiomyopathy and a decrease in the seizure threshold. Furthermore, in a recently published study by Tiihonen et al.27 (in which a 10-year follow-up was carried out for a Finnish cohort of 66,881 patients with a schizophrenia diagnosis), the authors concluded that the patients treated by clozapine had the lowest risk of premature death, compared with the patients who took other antipsychotics and the patients who took no antipsychotics regularly. This protective effect could be partially mediated by the reduction in suicide risk.28 The authors suggested that the restrictions on the use of clozapine “may have caused thousands of premature deaths.”
The fact that the majority of patients under clozapine treatment are resistant or have previously had significant adverse effects to other antipsychotics makes the decision to suspend clozapine treatment difficult. Many physicians consider reinitiating clozapine in patients with a history of neutropenia despite the risk that it might involve.29,30
Clozapine can be related to neutropenia both directly (as we previously examined) and indirectly. Examples of the latter include benign ethnic neutropenia, side effects of other drugs administered concomitantly or through interaction with clozapine and medical comorbidity.29 Methods described for the management of neutropenia in these patients include co-administering lithium, administering granulopoiesis stimulating factors and managing concomitant drugs rationally. While these treatments do provide a safety margin, they have been used in only a limited number of cases and the level of evidence is still weak.29,31,32
The protocols associated with clozapine use are extremely costly for the patient and for healthcare services. Current evidence available on the effectiveness and safety of this drug and the results of this study make it appropriate to start reviewing and discussing the protocols for clozapine usage and follow-up. Taking into consideration the greater risk concentrated in the first 6 months of treatment, and the risk of agranulocytosis after the first year–which is equal to that of other drugs not specifying monitoring–we would be in favour of maintaining a weekly follow-up in the first 18 weeks, a monthly follow-up throughout the rest of the first year and a bi-monthly follow-up after that. This change could involve a lower cost for the patient and for healthcare services and could also improve adherence to follow-ups and treatment.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflict of interestDr. Bernardo has received funds for research and/or has acted as a consultant and/or speaker in activities organised by the following companies: Adamet, Almirall, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Mylan, Pfizer, Rocher and Rovi.
Dr. Pons has acted as a consultant and/or speaker for the following companies: Janssen-Cilag and Johnson & Johnson.
We gratefully acknowledge the support of the Government of Cataluña, the Commission for Universities and the Development Office of the Department of Innovation, Universities and Enterprise (DIUE)2009SGR1295 and of the Carlos III Health Institute, Biomedical Research Centre in the Mental Health Network (CIBERSAM in English; for JU, AP, AB and MB) and with the support of the Josep Font Research Grant from the Clinical Hospital of Barcelona (for JU).
Please cite this article as: Pons A, et al. Clozapina y agranulocitosis en España: ¿tenemos una población más segura? Seguimiento hematológico a 5 años de una cohorte de pacientes tratados con clozapina. Rev Psiquiatr Salud Ment (Barc.). 2012;5(1):37–42.