The administration of multiple esketamine doses has shown efficacy for unipolar and bipolar treatment-resistant depression (TRD). Nevertheless, the probability of responding or not after each dose in the real-world remains unknown. This study aimed to estimate it throughout four doses of esketamine, administrated via subcutaneous (SC).
Material and methodsWe conducted a retrospective analysis of a case series of 70 patients with TRD who received treatment from the esketamine assistance program at Federal University of Sao Paulo, between April 2017 and December 2018. The SC injections were administrated weekly at a dose of 0.5–1.0mg/kg, in conjunction with patients’ psychotropic drugs. Response was defined as a decrease of at least 50% in the Montgomery-Åsberg Depression Rating Scale between baseline and 24h after dose. We used hidden Markov modeling in order to estimate de probability of response after each esketamine injection.
ResultsThe probability of a patient that was a “non-responder” to become a “responder” following a SC injection of esketamine was 17.30% and the probability that this patient remains a “non-responder” was 82.70%. The probability of a patient that was a “responder” to remain as a “responder” was 95%.
ConclusionsPatients with TRD who had not responded after the first dose of esketamine, still had a chance of responding after the subsequent dose administrated via SC.
La administración de dosis múltiples de esketamina ha demostrado su eficacia para el tratamiento de la depresión unipolar y bipolar resistente al tratamiento (TRD). Sin embargo, sigue siendo una incógnita la probabilidad de responder o no tras cada dosis en el mundo real. El objetivo de este estudio fue calcular dicha probabilidad durante la administración vía subcutánea (SC) de cuatro dosis de esketamina.
Material y métodosRealizamos un análisis retrospectivo de una serie de casos de 70 pacientes con TRD, que recibieron tratamiento a través del programa de asistencia con esketamina en la Universidad Federal University de Sao Paulo, entre abril de 2017 y diciembre de 2018. Las inyecciones SC se administraron semanalmente, a dosis de 0,5-1mg/kg, junto con los medicamentos psicotrópicos de los pacientes. Se definió la respuesta como una reducción de al menos el 50% en la Escala de Calificación de la Depresión de Montgomery-Åsberg entre el valor basal y las 24 horas posteriores a la administración de la dosis. Utilizamos el modelo oculto de Markov para calcular la probabilidad de respuesta tras cada inyección de esketamina.
ResultadosLa probabilidad de que un paciente que fuera «no respondedor» se convirtiera en «respondedor», tras una inyección SC de esketamina fue del 17,3%, y la probabilidad de que este paciente siguiera siendo «no respondedor» fue del 82,7%. La probabilidad de que un paciente que fuera «respondedor» lo siguiera siendo fue del 95%.
ConclusionesLos pacientes con TRD que no han respondido a la primera dosis de esketamina, tienen probabilidad de respuesta tras la administración de las siguientes dosis por vía SC.
The treatment of patients with bipolar depression or major depressive disorder may be very challenging, as pharmacological interventions are ineffective in a significant portion of cases.1 Patients who do not achieve remission after two or more treatments with an adequate dose of antidepressant for an adequate duration are considered to have treatment-resistant depression (TRD).2 Ketamine and esketamine have been shown to have a robust antidepressant effect in such cases and have been considered to be a breakthrough in this context.3–5 To date, there are no clinical practice guidelines recommending the use of ketamine or esketamine for depressive disorders.4
Ketamine is an N-methyl-d-aspartate (NMDA) receptor antagonist. Its antidepressant activity is attributed to several mechanisms that converge into the generation of synaptic plasticity and potentiating of excitatory neurotransmission.6 Most hypotheses consider the blockage of NMDA receptors to be essential to the antidepressant effect, but there is also research showing that ketamine's metabolites may independently exert it.7 At the cellular level, ketamine leads to the activation of post synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), that triggers intracellular brain-derived neurotrophic factor (BDNF) release and the subsequent activation of the mechanistic target of rapamycin complex 1 (mTORC1).8
Ketamine is a racemate, comprising the S-(1)-ketamine enantiomer (esketamine) and the R-(2)-ketamine enantiomer (arketamine). It has been demonstrated that esketamine is non-inferior compared to ketamine for patients with TRD.9 Esketamine has also been related to a better safety profile.10 It can be administrated through several routes, as following: oral, subcutaneous (SC), intranasal, intramuscular, intravenous (IV).11 Data with respect to SC administration of esketamine are limited.12 Although, it has been found that SC route appears to be more affordable and to have fewer collateral effects than the IV route, while showing comparable efficacy in the treatment of TRD.13
The administration of multiple doses of esketamine has shown efficacy for TRD, as demonstrated by a large number of published phase 2 and phase 3 studies.10,14–17 Nevertheless, these trials did not provide a clear idea regarding the probability that a “non-responder” will become a “responder” (and vice-versa) after each esketamine infusion. Generally, they reported an average odds ratio, calculated based on a longitudinal design and not on the individual transitions. Understanding the individual transitions should help us answer the following critical questions: if a patient is a “responder” to esketamine injection, what is the probability of maintaining the same response status after the second injection? Similarly, if a patient is a “non-responder” at first, what is the probability of becoming a “responder” after a subsequent dose? In the case of multiple esketamine injections, being a “responder” may impact the response status of the subsequent injection. Hidden Markov model (HMM) is applicable to the case of multiple esketamine injections as it calculates the probability of changing from one response status to another, while considering the previous status with each transition.
The current naturalistic cohort study examines the probability of transitioning from being a “non-responder” to becoming a “responder” after each SC administration of esketamine, following four injections from a series of six in 70 patients with TRD.
Material and methodsParticipants and proceduresThe data in this study originated from a retrospective analysis of a case series of 70 patients with TRD, who were referred by their treating psychiatrist to the esketamine clinic at the Department of Psychiatry of the Federal University of São Paulo, between April 2017 and December 2018.
Patients signed and dated a written informed consent document, in support of their participation in the study, and were aware they were not participating in a research protocol, but in an academic assistance program. The study was approved by the Federal University of São Paulo Ethics Committee (No. 434/2018).
Inclusion criteria were as follows: current diagnosis of either major depressive disorder or bipolar depression according to DSM-IV as assessed with the Mini-International Neuropsychiatric Interview-Plus (MINI-Plus) 5.0; prior history of non-response to at least two antidepressants or approved drugs for bipolar depression, used in an effective dose for at least 6 weeks; Montgomery-Åsberg Depression Rating Scale (MADRS) score of ≥25; age of 15 years or more. Exclusion criteria were as follows: history of hypersensitivity and/or allergy to ketamine/esketamine; diagnosis of ketamine/esketamine abuse or dependence; uncontrolled hypertension; pregnancy.
Participants were kept on stable doses of medications during the esketamine treatment. Each participant received up to six weekly SC injections of esketamine in the abdomen, at a dose of 0.5–1.0mg/kg. A response was defined as at least 50% decrease from baseline in the MADRS total score, 24h after dose administration.18 This definition was used to describe the patients as “responder” and “non-responder”. Patients, who did not become a “responder” within 24h of receiving a dose, received an increase of 0.25mg/kg in the subsequent dose, up to 1.0mg/kg.
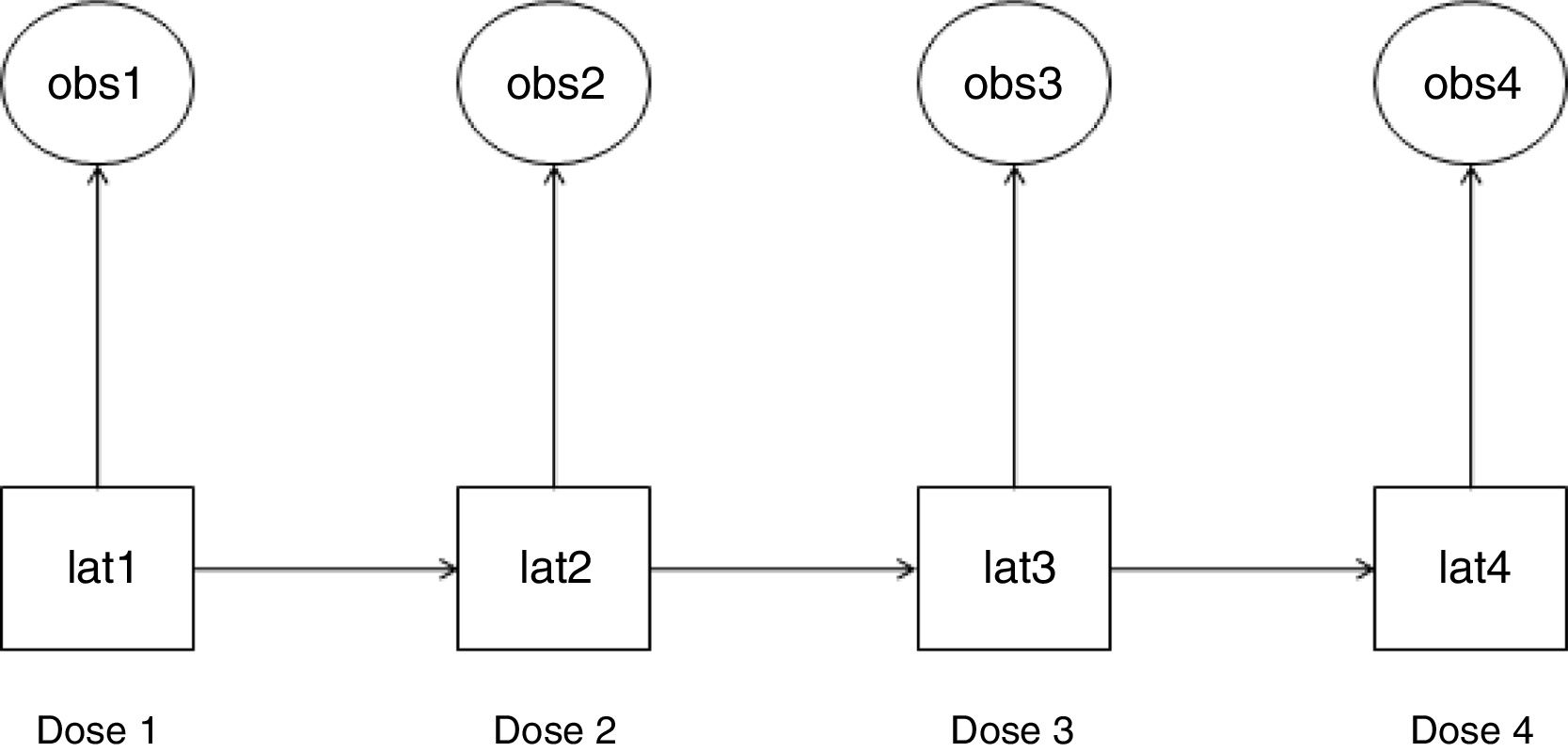
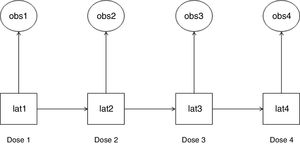
Statistical analysisWe used HMM to analyze the probability of a patient to become a “responder” or “non-responder” throughout four esketamine doses. This method permits the analysis of a system whose actual state is not yet known, but will be randomly originated from a certain future event.19 In this study, esketamine injection was the event and it generated two possible observed outcomes: “responder” and “non-responder”. As a latent transition analysis, HMM can describe the underlying population behavior based on a set of observed variables. It identifies latent class variables, which construct common patterns and behaviors from the whole population at the time of each of observation. HMM analyzes the way individuals transition longitudinally from one latent class to another,20 as illustrated in Fig. 1.
Hidden Markov model (HMM): observed variables and their corresponding latent class variables as they transition along the doses. The HMM generated a latent class variable for each time point after doses 1–4 (lat1, lat2, lat3, and lat4). Each latent class variable captured the unobserved features that were present at their respective time point and are correlated with the observed variable at that time point (obs1, obs2, obs3, and obs4). An observed variable and its corresponding latent class variable were either a “responder” or a “non-responder”. Each latent class variable transitioned into a subsequent one after the respective esketamine dose administration (e.g., lat1 transitioned into lat2 after dose 1).
Using Mplus 8 software,21 two HMMs were constructed. In one model, the transition matrices (latent state intercepts and autoregressive paths) were kept equal across the doses (Model 1). The other model had no constraints (Model 2). To calculate the difference in fit between the two models and to determine which model is more representative of the data, we applied scaled loglikelihood-ratio tests.
Additionally, we evaluated whether having major depressive disorder rather than bipolar depression would impact the latent response status across the doses of esketamine. Considering the reduced sample size and the number of parameters to estimate (one for each wave of assessment under HMM), the following constraints were imposed: we assumed that the probability of being “responder” and “non-responder” in every wave of assessment would be constant between both groups (i.e., having major depressive disorder rather than bipolar depression). Therefore, the hypothesis underlying these constraints is that having major depressive disorder would cause the same impact across the four injections, in terms of modifying the probability of response.
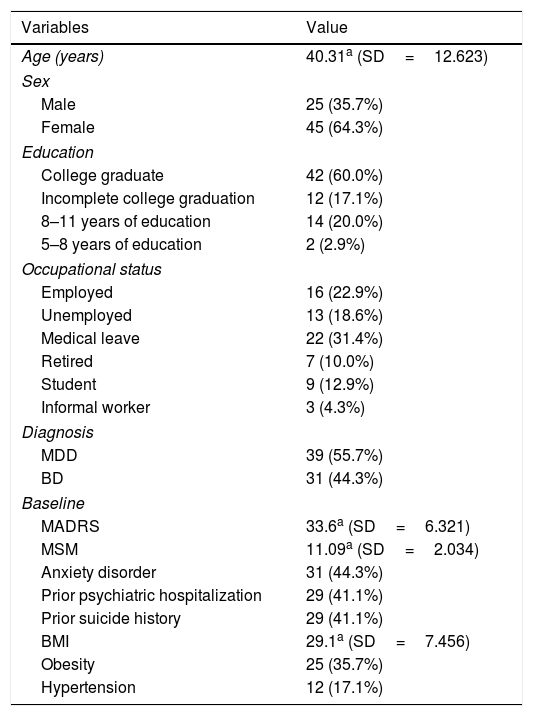
ResultsTable 1 shows the demographic and the clinical characteristics of the total cohort. This naturalistic cohort is representative of patients with TRD who are referred to off-label esketamine treatment. Most patients had severe TRD (mean Madsley Staging Method total score >11) and 81.42% of them had at least one clinical comorbidity.
Patient demographics and clinical characteristics.
| Variables | Value |
|---|---|
| Age (years) | 40.31a (SD=12.623) |
| Sex | |
| Male | 25 (35.7%) |
| Female | 45 (64.3%) |
| Education | |
| College graduate | 42 (60.0%) |
| Incomplete college graduation | 12 (17.1%) |
| 8–11 years of education | 14 (20.0%) |
| 5–8 years of education | 2 (2.9%) |
| Occupational status | |
| Employed | 16 (22.9%) |
| Unemployed | 13 (18.6%) |
| Medical leave | 22 (31.4%) |
| Retired | 7 (10.0%) |
| Student | 9 (12.9%) |
| Informal worker | 3 (4.3%) |
| Diagnosis | |
| MDD | 39 (55.7%) |
| BD | 31 (44.3%) |
| Baseline | |
| MADRS | 33.6a (SD=6.321) |
| MSM | 11.09a (SD=2.034) |
| Anxiety disorder | 31 (44.3%) |
| Prior psychiatric hospitalization | 29 (41.1%) |
| Prior suicide history | 29 (41.1%) |
| BMI | 29.1a (SD=7.456) |
| Obesity | 25 (35.7%) |
| Hypertension | 12 (17.1%) |
SD: standard deviation; MDD: Major Depressive Disorder; BD: Bipolar Disorder; MADRS: Montgomery–Åsberg Depression Rating Scale; MSM: Maudsley Staging Method; BMI: Body Mass Index. n; %
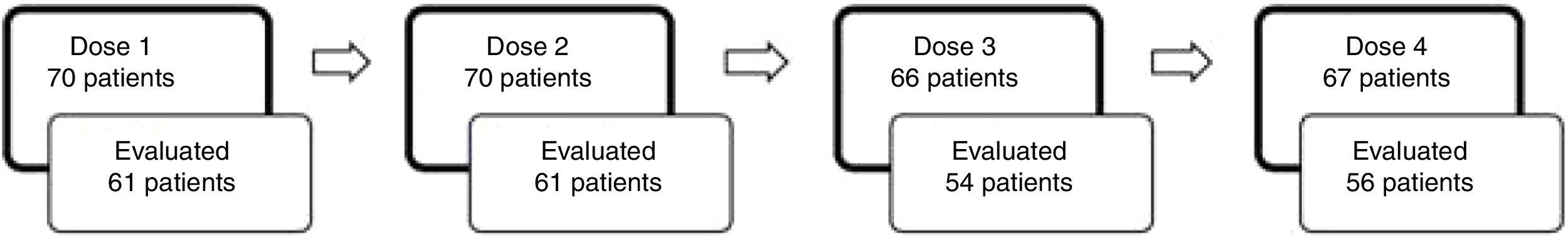
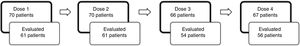
Fig. 2 illustrates the number of patients that received each dose of esketamine and the corresponding number of patients that were evaluated 24h after that dose. We observed that after dose 1, the proportion of patients responding to esketamine was 37.70%. After dose 4, it was 62.50%.
The constrained HMM (model 1) was more representative of the observed data than model 2 (freed HMM): Log likelihood: −137.84; test=χ2 (df) = 3.04, p-value=1.00. In model 1, a total of 16 patterns of the latent class variables were found across the doses. The most frequent patterns were: (a) “non-responder” across the four time points (46.44%), defined as “always non-responder” and (b) “responder” across the four time points (15.32%), defined as “always responder”. Other prevalent latent patterns were: non-responder-responder-responder-responder (12.85%); non-responder-non-responder-responder-responder (11.18%) and non-responder-non-responder-non-responder-responder (9.72%).
According to model 1, the probability of a patient that was a “non-responder” after dose 1 to become a “responder” following dose 2 was 17.30%, and the probability that this patient remains a “non-responder” following dose 2 was 82.70%. The probability of a patient that was a “responder” after dose 1 remains a “responder” following dose 2 was 95%, whereas the probability that this patient will move back to “non-responder” was 5%. Since model 1 has equal transitioning matrices, the same probabilities were obtained across the other doses.
We tested the hypothesis that patients with major depressive disorder would have a different probability of response across the doses when compared to bipolar depressive patients. Lack of evidence was found that unipolar depression would predict more responders (odds ratio=0.57, p-value=0.18).
DiscussionTo our knowledge, this is the first study that used HMM to estimate the real-world probability that TRD patients respond throughout multiple SC esketamine injections. As estimated by the best-fitted model, the patterns of “always non-responder” and “always responder” were the most frequent latent behaviors across the four doses; however, the “always non-responder” pattern was three times more frequent than the “always responder” pattern.
The model also found that the probability of being a “stayer” (maintaining the same latent response status of either “responder” or “non-responder”) from one injection to the subsequent one was higher than the probability of being of a “mover” (transitioning to a different latent status at any point). Accordingly, it appears to be more likely to maintain the latent status “responder” than to become a “responder”. Yet, the probability of moving from a “non-responder” to a “responder” after a dose was 17.30%. This probability is conditioned by the latent status obtained at the time before and, by constraints of the model, is held equal across the other time points.
Intranasal esketamine has been approved for the treatment of TRD, in conjunction with oral antidepressants.22 Even though the current study explored the SC route of administration, it provides information about repeated doses of esketamine in the clinical practice. We found no distinction in the probability of response to esketamine between unipolar and bipolar patients across the doses. On the contrary, a meta-analysis has shown that ketamine's antidepressant effect may extend over a longer period in the case of major depressive disorder compared to bipolar depression.23 Nevertheless, the study considered only trials with a single ketamine infusion. In clinical trials with multiple doses of ketamine, the number of patients who achieve response appears to increase in the case of unipolar depression24,25 and also in the case of bipolar depression.26
Our findings should be looked at with caution because the study has some limitations. The absence of a control group and randomization, which are inherent to a real-world analysis, limits the internal validity of the study. Patients came from the same academic site, and the number of patients enrolled in the study was somewhat limited. In addition, some of the patients missed one or more evaluations, which is common in a real-world study. The number of patients has also limited the analysis of whether having major depression disorder would be a modifier of the probability of response to esketamine compared to having bipolar depression. Moreover, participants kept their existing psychotropic medications during the esketamine treatment, which may have influenced the results. Furthermore, we did not consider the increase in the esketamine dose as a variable and the response was evaluated over a short period of time.
In conclusion, our results provide details about the transition dynamics of response to multiple SC doses of esketamine in the treatment of TRD. Considering four SC esketamine injections, a patient with TRD, who had been “non-responder” after a dose, still had a chance (17.30%) to become a “responder” following the subsequent dose. Additional research with a larger sample size a randomized placebo-controlled design is needed to further evaluate the response to multiple doses of esketamine administered through the SC route.
FundingThis work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Conflict of interestThe authors declare the following potential conflicts of interest:
Dr. Sarin reports personal fees from Daiichi Sankyo Brasil, Lundbeck Brasil, Pfizer, and Janssen and non-financial support from Takeda Brasil, Moksha8 Brasil, and Torrent Pharma, outside the submitted work. Dr. Magalhães reports non-financial support from Torrent Pharma and Hypera Pharma, outside the submitted work. Dr. Nakahira reports non-financial support from Eurofarma, Cristália, and Sanofi, outside the submitted work. Dr. Lacerda has received consulting fees from Janssen Pharmaceutical, Daiichi Sankyo Brasil, Cristalia Produtos Químicos e Farmacêuticos, Pfizer, Mantecorp Indústria Química e Farmacêutica, Libbs Farmacêutica, and Sanofi-Aventis over the last 24 months and has received research fees from Janssen Pharmaceutical, Eli Lilly, H. Lundbeck A/S, Servier Laboratories, Hoffman-La Roche, and Forum Pharmaceuticals, not related to the submitted manuscript. None of the remaining authors have any potential conflicts to disclose.