Immune system plays an important role in behavior of cancer. Breast cancer and its stroma display high levels of immune-cell infiltrates (tumor-infiltrating lymphocytes TILs). Programmed cell death-ligand 1 (PD-L1) is one key inhibitor mechanism for TILs. Potential of TILs and PD-L1 as predictive factors for chemotherapy response in breast cancer is a promising field of study. The aim of this work is to investigate the correlation of PD-L1, TILs and pathologic response following neoadjuvant chemotherapy (NAC) in breast cancer patients and effect of NAC on PD-L1 expression and TILs in residual tumor mass.
MethodsThirty patients with invasive duct carcinoma diagnosed by core biopsy and received NAC were enrolled in this prospective study from November 2018 to October 2020. PD-L1 expression was evaluated by immunohistochemistry and relates this to TILs, clinical characteristics, and response to neoadjuvant chemotherapy and then re-evaluated in residual masses after surgery.
ResultsPD-L1 expression was observed in 40% of cases in the tumor cells. High stromal TILs were detected in 46.7%. Also, 64.3% and 58.3% of patients expressed TILs and PD-L1 (respectively) in their core biopsies gained complete clinical and pathologic responses with significant difference (p value<0.001; 0.013). High PD-L1 expression and high TILs were observed in triple negative breast cancer (TNBC) and Her2 enriched cases but without significant difference.
ConclusionHigh TILs and PD-L1 are associated with complete clinical and pathologic responses in breast cancer patients who receiving NAC.
El sistema inmune juega un papel importante en el comportamiento del cáncer. El cáncer de mama y su estroma exhiben altos niveles de infiltrados de las células inmunitarias (linfocitos infiltrantes de tumor [TIL]). El ligando del factor de muerte programada 1 (PD-L1) es un mecanismo inhibidor clave para los TIL. El potencial de TIL y PDL-1 como factores predictivos para la respuesta a la quimioterapia en el cáncer de mama es un campo de estudio prometedor. El objetivo de este estudio es estudiar la correlación de PD-L1, TIL y la respuesta patológica tras la quimioterapia coadyuvante (NAC) en pacientes con cáncer de mama, así como el efecto de NAC en la expresión de PDL-1 y TIL en la masa tumoral residual.
MétodosSe incluyó en este estudio prospectivo a 30 pacientes con carcinoma ductal invasivo diagnosticadas mediante biopsia central, que recibieron NAC de noviembre de 2018 a octubre de 2020. La expresión de PD-L1 fue evaluada mediante inmunohistoquímica, y fue relacionada con TIL, características clínicas y respuesta a la quimioterapia neoadyuvante, reevaluándose en masas residuales tras la cirugía.
ResultadosLa expresión de PD-L1 se observó en el 40% de los casos en las células tumorales. Se detectaron altos niveles de TIL estromales en el 46,7% de los casos. De igual modo, el 64,3 y el 58,3% de las pacientes con expresión de TIL y PDL-1, respectivamente, en sus biopsias centrales logró respuestas clínicas y patológicas completas con diferencia significativa (p<0,001; 0,013). La alta expresión de PDL1 y los altos niveles de TIL fueron observados en el cáncer de mama triple negativo (TNBC) y en los casos de Her2 enriquecido, aunque sin diferencia significativa.
ConclusiónLa alta expresión de TIL y PDL1 se asocia a respuestas clínicas y patológicas completas en las pacientes de cáncer de mama que reciben NAC.
Tumor infiltrating lymphocytes (TILs) are assumed to be closely related to the proliferation and elimination of cancer cells. Furthermore, the state of expression of TILs in situ has been identified as a useful factor for predicting responses to chemotherapy, and has been attracting attention as a new biological marker in breast cancer. PD-L1 is T-lymphocyte-inhibitory molecule acts by binding of programmed cell death protein-1 (PD-1) on lymphocytes and programmed cell death-ligand 1 (PD-L1) on cancer cells.1
Therefore, these are most useful when assessed before the initiation of treatment with neoadjuvent chemotherapy (NAC). Some types of aggressive breast cancer do not respond to hormonal therapy such as hormonal negative and triple negative (TNBC) breast cancer. PD-L1 expression in TNBC has been shown to range from 40 to 65% in several studies.2
Our goal was to investigate the correlation of PD-L1, also known as B7-H1 and CD274, with TILs and pathologic response (pCR) following neoadjuvant chemotherapy in breast cancer. We assess PD-L1 expression objectively on samples from a cohort of patients with breast cancer that received neoadjuvant chemotherapy and relate this to TILs, clinical characteristics of the cancer, and response to neoadjuvant chemotherapy.
Material and methodsPatients and specimen selectionThirty patients with invasive duct carcinoma (NST) were enrolled in this prospective study, during the period from November 2018 to October 2020. This study was carried out at clinical oncology, pathology, Oncology unit of General surgery departments, Faculty of Medicine, Zagazig University, Egypt. The diagnosis of breast cancer was achieved thorough clinical examination followed by mammography, ultrasonography, and eventually a core biopsy. Institutional Review Board (IRB) of the faculty of Medicine Zagazig University confirmed the study protocol (No. 5737). Written informed consent was obtained from all participants.
Evaluation of response to neoadjuvant chemotherapyClinical response to NAC was evaluated using clinical and ultrasonographic examination to breast, a clinical complete response (CR) occurred when there was complete disappearance of the palpable primary tumor masses. Clinical partial response (PR) occurs if >50% reduction in tumor volume. Tumor reduction <50% or an increase in the size up to 25% was considered as stable disease (SD). An increase of >25% of tumor size was named a progressive disease (PD). Pathological response was complete response or residual disease. Pathological complete response (pCR) was the absence of invasive tumor in the breast and axillary nodes.
Immunohistochemistry procedureImmunohistochemical staining was carried out using the polymer Envision detection system; the Dako EnVision™ kit (Dako, Copenhagen, Denmark). Tissue sections (3–5μm) were deparaffinized in xylene and rehydrated in graded alcohol. To block endogenous peroxidase, slides were incubated for 10min in 3% hydrogen peroxide. Dako target antigen retrieval solution (pH 6.0) was applied for 20min. Then slides were incubated for 60min with a primary polyclonal PD-L1 antibody (Abcam, ab58810, polyclonal, 1:200 dilutions, Cambridge, UK). The reaction was visualized by incubating the sections with diaminobenzidine (DAB) for 15min after that Mayer's hematoxylin was used.
Interpretation of immunostainingPD-L1 was considered positive when tumor cells with partial or complete cell membrane staining or cytoplasmic staining. PD-L1 positivity will be defined as >1% of positive tumor cells.3
Evaluation of tumor-infiltrating lymphocytes (TILs)TILs were assessed in hematoxylin and eosin-stained sections, carefully following the guidelines published by the International TILs Working Group to standardize TILs evaluation. Cases were defined as; high TILs (lymphocytic predominant breast cancer) in tumors with ≥50% of stromal surface areas showed lymphocytic infiltration and as; low TILs for <50%.4
Statistical analysisAll data were collected, tabulated and statistically analyzed using SPSS 22.0 for windows (IBM Inc., Chicago, IL, USA). Continuous variables were expressed as the mean±SD and median (range), and the categorical variables were expressed as a number (percentage). Continuous variables were checked for normality by using Shapiro–Wilk test. Wilcoxon signed ranks test was used to compare two dependent groups of non-normally distributed variables. Stuart–Maxwell test was used to test change in multinomial categorical data of two samples. Mann–Whitney U test was used to compare between two groups of non-normally distributed variables. Categorical data were compared using Chi-square test or Fisher's exact test when appropriate. All tests were two sided. p-Value <0.05 was considered statistically significant (S), p-value <0.001 was considered highly statistically significant (HS), and p-value ≥0.05 was considered statistically insignificant (NS).
ResultsPatients’ characteristicsThe clinicopathologic parameters of the studied cases (N=30). The mean age at presentation was 46.66±12.55 years, with range from 26 to 75 years. All cases were high grade (2–3) invasive duct carcinoma (NOS) and 26.7% of the cases showed lymphovascular invasion. As regards the pathologic stage, 30 cases were pT2. Specimens taken were 30 core biopsies from patients before receiving neoadjuvant chemotherapy protocol (AC-paclitaxel); 4 cycles of doxorubicin (60mg/m2 day1/21 days) and cyclophosphamide (600mg/m2 day1/21days) followed by 4 cycles of paclitaxel (175mg/m2 IV. 3h day1/21 days). In this study, we excluded all other special types of breast cancer, patients who have other malignancies, patients who ineligible for neoadjuvant chemotherapy. The clinico-pathological data were collected by oncologists and pathologists. The tumors were graded according to the Nottingham modification of the Bloom–Richardson system. The ER, PR, and HER2 staining were obtained as described in patients’ reports. Molecular classification of patients was selected as follows: 5 luminal A, 12 luminal B, 5 triple-negative and 8 HER2-neu enriched type. Nine cases (30%) achieved complete pathological response (pCR) while 21 (70%) were less than (pCR). Seven patients had breast conservative surgery while 23 had modified radical mastectomy (Table 1).
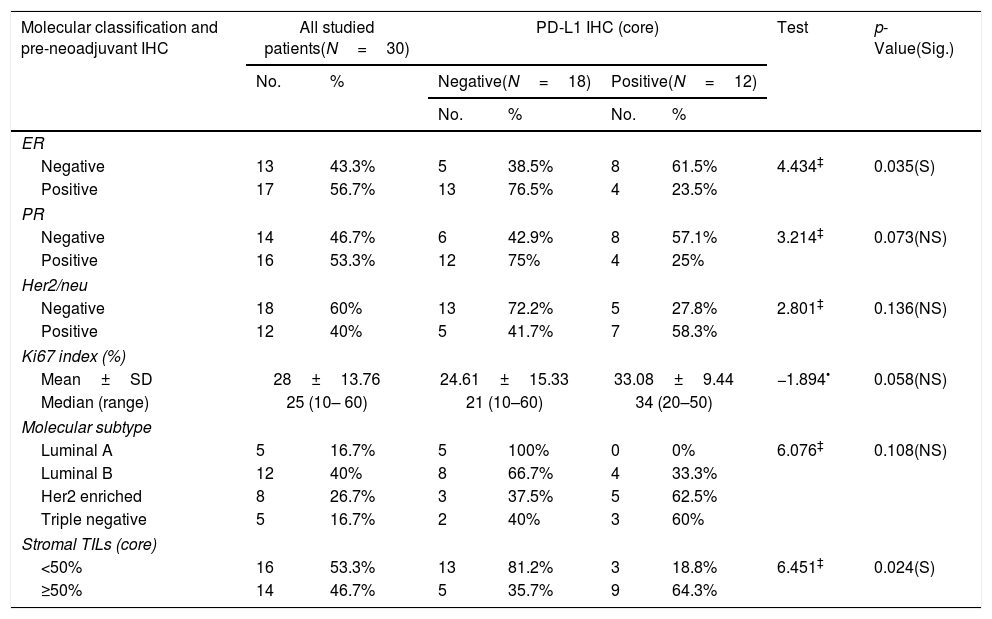
Relationship between molecular classification/pre-neoadjuvant immunohistochemistry and PD-L1 IHC (core) among the studied breast cancer patients (N=30).
| Molecular classification and pre-neoadjuvant IHC | All studied patients(N=30) | PD-L1 IHC (core) | Test | p-Value(Sig.) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | Negative(N=18) | Positive(N=12) | |||||
| No. | % | No. | % | |||||
| ER | ||||||||
| Negative | 13 | 43.3% | 5 | 38.5% | 8 | 61.5% | 4.434‡ | 0.035(S) |
| Positive | 17 | 56.7% | 13 | 76.5% | 4 | 23.5% | ||
| PR | ||||||||
| Negative | 14 | 46.7% | 6 | 42.9% | 8 | 57.1% | 3.214‡ | 0.073(NS) |
| Positive | 16 | 53.3% | 12 | 75% | 4 | 25% | ||
| Her2/neu | ||||||||
| Negative | 18 | 60% | 13 | 72.2% | 5 | 27.8% | 2.801‡ | 0.136(NS) |
| Positive | 12 | 40% | 5 | 41.7% | 7 | 58.3% | ||
| Ki67 index (%) | ||||||||
| Mean±SD | 28±13.76 | 24.61±15.33 | 33.08±9.44 | −1.894• | 0.058(NS) | |||
| Median (range) | 25 (10– 60) | 21 (10–60) | 34 (20–50) | |||||
| Molecular subtype | ||||||||
| Luminal A | 5 | 16.7% | 5 | 100% | 0 | 0% | 6.076‡ | 0.108(NS) |
| Luminal B | 12 | 40% | 8 | 66.7% | 4 | 33.3% | ||
| Her2 enriched | 8 | 26.7% | 3 | 37.5% | 5 | 62.5% | ||
| Triple negative | 5 | 16.7% | 2 | 40% | 3 | 60% | ||
| Stromal TILs (core) | ||||||||
| <50% | 16 | 53.3% | 13 | 81.2% | 3 | 18.8% | 6.451‡ | 0.024(S) |
| ≥50% | 14 | 46.7% | 5 | 35.7% | 9 | 64.3% | ||
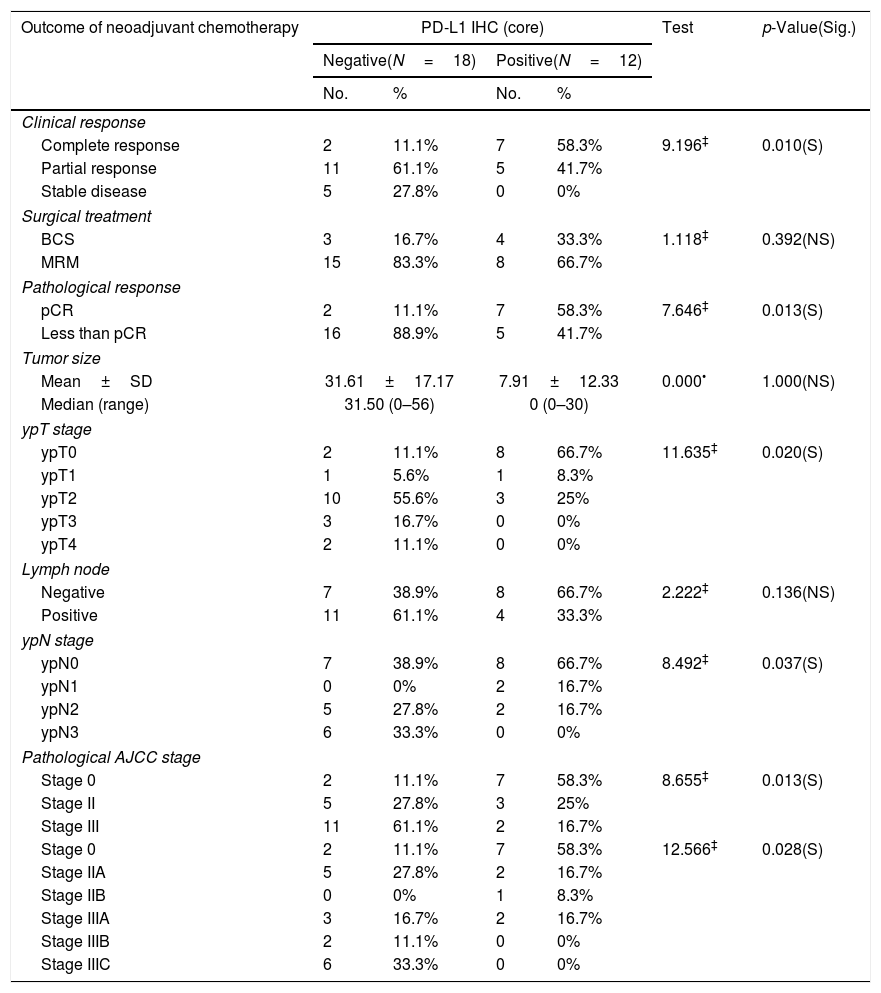
PD-L1 expression was observed in 40% (12/30) cases in the tumor cells of core biopsies. Stromal TILs were detected in H&E slide sections in 46.7% (14/30) cases. Table 4 shows 64.3% and 58.3% of patients expressed high TILs and PD-L1 (respectively) in their core biopsies gained complete clinical and pathologic response. PD-L1 expression was detected in high stage breast cancer (III) with significant difference (p value<0.02). Cases with positive PD-L1 expression gave complete clinical and pathologic responses with significant differences (p value<0.010, 0.013 respectively) (Tables 2 and 4).
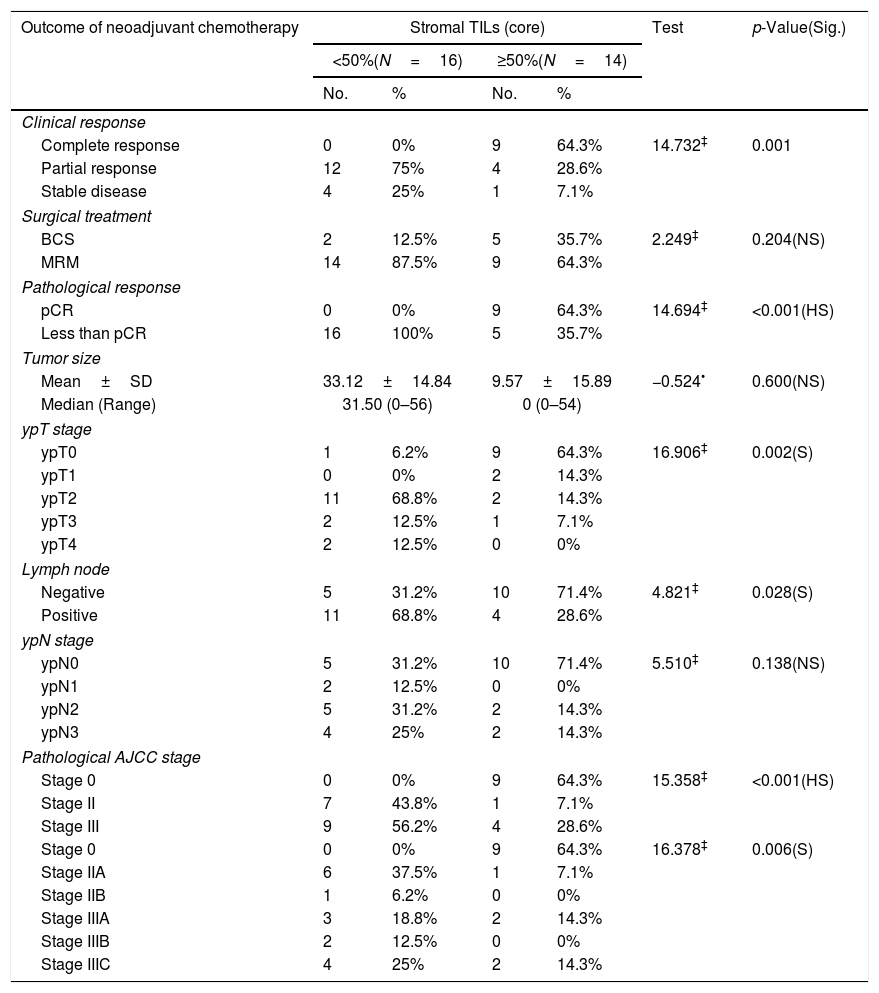
Relationship between stromal TILs IHC (core) and outcome of neoadjuvant chemotherapy among the studied breast cancer patients (N=30).
| Outcome of neoadjuvant chemotherapy | Stromal TILs (core) | Test | p-Value(Sig.) | |||
|---|---|---|---|---|---|---|
| <50%(N=16) | ≥50%(N=14) | |||||
| No. | % | No. | % | |||
| Clinical response | ||||||
| Complete response | 0 | 0% | 9 | 64.3% | 14.732‡ | 0.001 |
| Partial response | 12 | 75% | 4 | 28.6% | ||
| Stable disease | 4 | 25% | 1 | 7.1% | ||
| Surgical treatment | ||||||
| BCS | 2 | 12.5% | 5 | 35.7% | 2.249‡ | 0.204(NS) |
| MRM | 14 | 87.5% | 9 | 64.3% | ||
| Pathological response | ||||||
| pCR | 0 | 0% | 9 | 64.3% | 14.694‡ | <0.001(HS) |
| Less than pCR | 16 | 100% | 5 | 35.7% | ||
| Tumor size | ||||||
| Mean±SD | 33.12±14.84 | 9.57±15.89 | −0.524• | 0.600(NS) | ||
| Median (Range) | 31.50 (0–56) | 0 (0–54) | ||||
| ypT stage | ||||||
| ypT0 | 1 | 6.2% | 9 | 64.3% | 16.906‡ | 0.002(S) |
| ypT1 | 0 | 0% | 2 | 14.3% | ||
| ypT2 | 11 | 68.8% | 2 | 14.3% | ||
| ypT3 | 2 | 12.5% | 1 | 7.1% | ||
| ypT4 | 2 | 12.5% | 0 | 0% | ||
| Lymph node | ||||||
| Negative | 5 | 31.2% | 10 | 71.4% | 4.821‡ | 0.028(S) |
| Positive | 11 | 68.8% | 4 | 28.6% | ||
| ypN stage | ||||||
| ypN0 | 5 | 31.2% | 10 | 71.4% | 5.510‡ | 0.138(NS) |
| ypN1 | 2 | 12.5% | 0 | 0% | ||
| ypN2 | 5 | 31.2% | 2 | 14.3% | ||
| ypN3 | 4 | 25% | 2 | 14.3% | ||
| Pathological AJCC stage | ||||||
| Stage 0 | 0 | 0% | 9 | 64.3% | 15.358‡ | <0.001(HS) |
| Stage II | 7 | 43.8% | 1 | 7.1% | ||
| Stage III | 9 | 56.2% | 4 | 28.6% | ||
| Stage 0 | 0 | 0% | 9 | 64.3% | 16.378‡ | 0.006(S) |
| Stage IIA | 6 | 37.5% | 1 | 7.1% | ||
| Stage IIB | 1 | 6.2% | 0 | 0% | ||
| Stage IIIA | 3 | 18.8% | 2 | 14.3% | ||
| Stage IIIB | 2 | 12.5% | 0 | 0% | ||
| Stage IIIC | 4 | 25% | 2 | 14.3% | ||
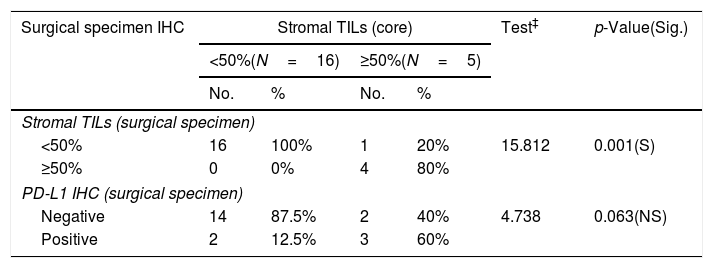
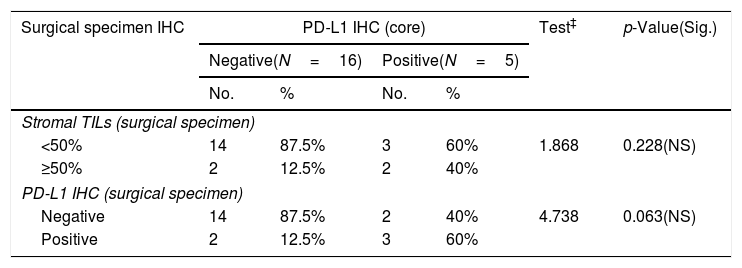
Numbers of patients with residual tumor masses were 21 patients while 9 patients gave complete pathologic response to NAC. PD-L1 expression in surgical specimens was 23.8%; and high TILs in 19.05% of cases. There was a near significant correlation between PD-L1 expression in core specimen and surgical biopsy specimens (p value<0.063) (Tables 3 and 5).
Relationship between stromal TILs IHC (core) and surgical specimen immunohistochemistry among the studied breast cancer patients (N=30).
| Surgical specimen IHC | Stromal TILs (core) | Test‡ | p-Value(Sig.) | |||
|---|---|---|---|---|---|---|
| <50%(N=16) | ≥50%(N=5) | |||||
| No. | % | No. | % | |||
| Stromal TILs (surgical specimen) | ||||||
| <50% | 16 | 100% | 1 | 20% | 15.812 | 0.001(S) |
| ≥50% | 0 | 0% | 4 | 80% | ||
| PD-L1 IHC (surgical specimen) | ||||||
| Negative | 14 | 87.5% | 2 | 40% | 4.738 | 0.063(NS) |
| Positive | 2 | 12.5% | 3 | 60% | ||
Relationship between PD-L1 IHC (core) and outcome of neoadjuvant chemotherapy among the studied breast cancer patients (N=30).
| Outcome of neoadjuvant chemotherapy | PD-L1 IHC (core) | Test | p-Value(Sig.) | |||
|---|---|---|---|---|---|---|
| Negative(N=18) | Positive(N=12) | |||||
| No. | % | No. | % | |||
| Clinical response | ||||||
| Complete response | 2 | 11.1% | 7 | 58.3% | 9.196‡ | 0.010(S) |
| Partial response | 11 | 61.1% | 5 | 41.7% | ||
| Stable disease | 5 | 27.8% | 0 | 0% | ||
| Surgical treatment | ||||||
| BCS | 3 | 16.7% | 4 | 33.3% | 1.118‡ | 0.392(NS) |
| MRM | 15 | 83.3% | 8 | 66.7% | ||
| Pathological response | ||||||
| pCR | 2 | 11.1% | 7 | 58.3% | 7.646‡ | 0.013(S) |
| Less than pCR | 16 | 88.9% | 5 | 41.7% | ||
| Tumor size | ||||||
| Mean±SD | 31.61±17.17 | 7.91±12.33 | 0.000• | 1.000(NS) | ||
| Median (range) | 31.50 (0–56) | 0 (0–30) | ||||
| ypT stage | ||||||
| ypT0 | 2 | 11.1% | 8 | 66.7% | 11.635‡ | 0.020(S) |
| ypT1 | 1 | 5.6% | 1 | 8.3% | ||
| ypT2 | 10 | 55.6% | 3 | 25% | ||
| ypT3 | 3 | 16.7% | 0 | 0% | ||
| ypT4 | 2 | 11.1% | 0 | 0% | ||
| Lymph node | ||||||
| Negative | 7 | 38.9% | 8 | 66.7% | 2.222‡ | 0.136(NS) |
| Positive | 11 | 61.1% | 4 | 33.3% | ||
| ypN stage | ||||||
| ypN0 | 7 | 38.9% | 8 | 66.7% | 8.492‡ | 0.037(S) |
| ypN1 | 0 | 0% | 2 | 16.7% | ||
| ypN2 | 5 | 27.8% | 2 | 16.7% | ||
| ypN3 | 6 | 33.3% | 0 | 0% | ||
| Pathological AJCC stage | ||||||
| Stage 0 | 2 | 11.1% | 7 | 58.3% | 8.655‡ | 0.013(S) |
| Stage II | 5 | 27.8% | 3 | 25% | ||
| Stage III | 11 | 61.1% | 2 | 16.7% | ||
| Stage 0 | 2 | 11.1% | 7 | 58.3% | 12.566‡ | 0.028(S) |
| Stage IIA | 5 | 27.8% | 2 | 16.7% | ||
| Stage IIB | 0 | 0% | 1 | 8.3% | ||
| Stage IIIA | 3 | 16.7% | 2 | 16.7% | ||
| Stage IIIB | 2 | 11.1% | 0 | 0% | ||
| Stage IIIC | 6 | 33.3% | 0 | 0% | ||
Relationship between PD-L1 IHC (core) and surgical specimen immunohistochemistry among the studied breast cancer patients (N=30).
| Surgical specimen IHC | PD-L1 IHC (core) | Test‡ | p-Value(Sig.) | |||
|---|---|---|---|---|---|---|
| Negative(N=16) | Positive(N=5) | |||||
| No. | % | No. | % | |||
| Stromal TILs (surgical specimen) | ||||||
| <50% | 14 | 87.5% | 3 | 60% | 1.868 | 0.228(NS) |
| ≥50% | 2 | 12.5% | 2 | 40% | ||
| PD-L1 IHC (surgical specimen) | ||||||
| Negative | 14 | 87.5% | 2 | 40% | 4.738 | 0.063(NS) |
| Positive | 2 | 12.5% | 3 | 60% | ||
Tumor microenvironment is the mirror that reflects survival, progression and invasion or metastasis of the cancer. Normal breast tissue contains few aggregates of immune cells but of no reported value, but breast cancer displays higher levels of immune cell infiltrates which play an important role to fight cancer. GEPARDUO and GEPARTRIO trials demonstrated that patient with higher TILs had greater rate of pCR (40%).5
PD-L1 (B7-1) a cell surface glycoprotein, is expressed by antigen presenting cells and can induce T-lymphocyte anergy through ligation with its T-lymphocyte receptors PD1 so it can protect tumor cells from lysis and death by immune system.6,7 PD1/PD-L1 axis is one of the immune modulatory pathways that inhibit TILs antitumor action, so inhibition of PD1/PD-L1 axis may enhance antitumor immunity and promote tumor regression.8 Our study started from this point to correlate TILs and PD-L1 expression in the tumor micro environment and response to neoadjuvant chemotherapy in different molecular subtypes of breast cancer.
As regards the immunohistochemical expression of PD-L1 in tumor cells and high TILs among the studied cases in the present study; PD-L1 reported in 40% of cases and high TILs 46.6%. In keeping with these results, in previous breast cancer studies PD-L1 expression was described between 15.8% and 37%,9,10 high TILs between 43% and 54%.11,12
The recognized association between PD-L1, TILs and unfavorable clinicopathological features is explained by activation of the PD-1/PD-L1 axis, thereby allowing tumor cells to escape from antitumor immune surveillance, proliferate and spread more rapidly.13
Concerning the association between PD-L1 positive tumor cells, stromal TILs density with the molecular subtypes; high TILs was reported in 80% of TNBC, 62.5% of HER enriched tumors. As well as high PD-L1 was in 60% of TNBC, 62.5% of HER 2 enriched tumor cells suggesting its clinical significance to be used as targeted therapy in hormone negative patients.
Breast cancer was considered a less immunogenic neoplasm as compared with other tumors. However, anti-PD-L1 monoclonal antibodies are encountered as a novel immunotherapy in invasive duct carcinoma especially in TNBC.14 This study highlights the major role of the immune system in the prognosis of breast cancer and the interaction between chemotherapy and the immune system in this setting.
In this study 64.3% of cases with high stromal TILs, 58.3% of patients with PD-L1 expression in their core biopsies showed complete clinical and pathological response to neoadjuvant treatment. Many prospective studies confirmed that higher pretreatment stromal TILs count and presence of PD-L1 expression are associated with higher probability of pCR. These studies confirmed the value of both TILs and PD-L1 as additional parameters in predicting response to neoadjuvant chemotherapy and as a promising therapeutic strategy.15,16
So, the immunogenic signature consisting of high TILs and PD-L1 expression in pretreatment breast cancer tissue that increased chance for pathologic complete response provides rationale for adding immune check point inhibitor to chemotherapy to increase PCR rate especially in the high immunogenic triple negative and HER-2 positive breast cancer subtypes.
Concerning the availability of post treatment tissue, chemotherapy induced changes in TILs and PD-L1 expression were documented. The anticipated finding was increase in TILs and PD-L1 expression in post treatment tissue depending on the validated data about positive immunogenic effect of chemotherapy on breast cancer tissue.17 However we observed decrease TILs and PD-L1 expression but without significant results between pretreatment and post treatment tissues. As most cases with less than pathologic complete response was luminal subtypes which explain the weak interaction or effect of chemotherapy on the cancer tissue and its immunogenic profile.
Our study highlights that correlation between biologic subtype of breast cancer, and immunogenic profile is promising signature in expecting the eligible breast cancer patient for neoadjuvant systemic therapy either chemotherapy alone or combined with anti PD-L1 immunotherapy. While this is a prospective study it depends on new cases diagnosed as breast cancer and were legible to receive neoadjuvent chemotherapy so the small sample size of our work may represent an obstacle to get more powerful results.
ConclusionFinally, as conclusions of this study, pathologic complete response to neoadjuvant chemotherapy is associated with an immunologic profile of breast cancer. High TILs and PD-L1 are associated with complete clinical and pathologic responses in breast cancer patients who receiving NAC.
FundingThis research received no specific grant from any funding agency in the public,commercial, or not-for-profit sectors.
Ethical considerationInstitutional Review Board (IRB) of the faculty of Medicine Zagazig University confirmed the study protocol (No. 5737). Written informed consent was obtained from all participants.
Conflict of interestsThe authors declare that they have no conflict of interest.