The use of toxic substances is a main risk factor for sudden cardiac death. The most relevant toxic substances are the illegal drugs (especially cocaine), ethanol, tobacco, and doping substances. Additionally, several therapeutic drugs are able to increase the risk for sudden cardiac death. The aim of the present paper is to review the mechanism of action and the main pathological problems induced by toxic substances. Moreover, we provide epidemiological information, underlie the importance of a standardised forensic investigation, and discuss the role of forensic pathology in the prevention of this phenomenon. The possibility of consumption of any drug of abuse should be considered in any case of sudden cardiac death in adolescents, young, or middle-age patients, especially in men. In athletes the use of doping substances should be ruled-out. In patients under psychopharmacological treatment, the putative influence of these drugs should be borne in mind.
El consumo de sustancias tóxicas constituye un factor de riesgo significativo para la muerte súbita cardiaca. Los tóxicos con mayor relevancia son las drogas ilegales (especialmente la cocaína), el alcohol y el tabaco, y en menor medida, las sustancias dopantes. Diversos medicamentos también incrementan el riesgo de muerte súbita cardiaca. En el presente artículo se hace una revisión sobre el mecanismo de acción y la patología inducida por estos tóxicos; se ofrece información epidemiológica, se destaca la trascendencia de una investigación forense protocolizada y se discuten las implicaciones de la patología forense en la prevención. La posibilidad de consumo de drogas de abuso debería ser tenida en cuenta en toda muerte súbita cardiaca de un adolescente, joven o adulto de edad mediana, principalmente en varones. En deportistas sería conveniente descartar el uso de sustancias dopantes; y en pacientes que toman psicofármacos valorar su influencia en el fallecimiento, aún con niveles terapéuticos.
The use of toxic substances has been associated with the onset of both acute and chronic health problems. The harmful effects are particularly important for the cardiovascular system, and are a significant risk factor for sudden cardiac death (SCD). Toxic substances, through transient functional disturbances, interact with the anatomical substrate responsible for SCD, triggering a fatal arrhythmia. In addition, toxic substances can cause chronic structural abnormalities, through sympathomimetic or direct cardiotoxic effects, which also increase the risk of SCD.
In the field of forensic pathology, relevant toxic substances are not only illegal drugs, but also ethyl alcohol, tobacco and performance-enhancing drugs. Although they cannot strictly be considered toxic substances, several medicinal products also increase the risk of SCD, particularly psychotropic drugs.
Few studies based on forensic autopsies have analysed the association between SCD and the use of toxic substances.1–3 In Seville, 668 sudden deaths were analysed; 21 of which were associated with the recent use of cocaine.1 In a case–control study conducted in the Spanish province of Biscay, 311 SCDs were examined in people aged between 15 and 49 years. The rate of recent drug use was as follows: alcohol 13%, benzodiazepines 10%, cocaine and cannabis 7%, opioids 4% and amphetamines 3%. Smoking accounted for 42%. The main risk factor for SCD was the recent use of cocaine (risk multiplied fourfold), followed by smoking (risk increased twofold).2 In a study carried out in Denmark on 477 cases of SCD in which toxicological analyses were performed, it was revealed that 57% of the cases were positive for some toxic substance or drug, with the following results in descending order: benzodiazepines (n=133), opioid agonists (n=103), ethanol (n=97), antidepressants (n=80), cannabis (n=43), cocaine (n=13), amphetamines (n=13) and gamma-hydroxybutyrate (n=5).3
The prevalence of SCD associated with toxic substances is not well defined and the pathophysiological mechanisms are not clearly established. Controlled epidemiological studies based on forensic autopsies are required in order to better understand this phenomenon. Forensic research provides a rich contribution to the understanding of these deaths for several reasons: (a) it provides additional evidence to the information collected in studies based on hospital populations or emergencies; (b) it allows reliable epidemiological figures to be obtained; (c) it allows both histopathological and toxicological studies to be carried out, offering reliable data which cannot be determined in clinical studies; and (d) it enables a chronological pattern to be established between the use of the substance and the pathological findings observed.
This article reviews the association between the use of toxic substances and SCD, providing data on the mechanism of action and the condition induced by the main substances. In addition, it offers epidemiological information, highlights the significance of a standardised forensic investigation and discusses the implications of forensic pathology in preventing this phenomenon.
Epidemiology of toxic substance useIt is estimated that around 25% of the adult population in the European Union has used illegal drugs at some point in their lives.4 This use is more common in men than in women, and is greater among people aged 15–34 years. In general, it entails polydrug use, involving the use of different substances. The most used illegal drug is cannabis, followed by various stimulants, such as cocaine and amphetamine derivatives, the use of which is significantly lower.4 Although the prevalence of its use is lower compared to other types of substances, opioid use is still responsible for a significant proportion of drug-related morbidity and mortality.
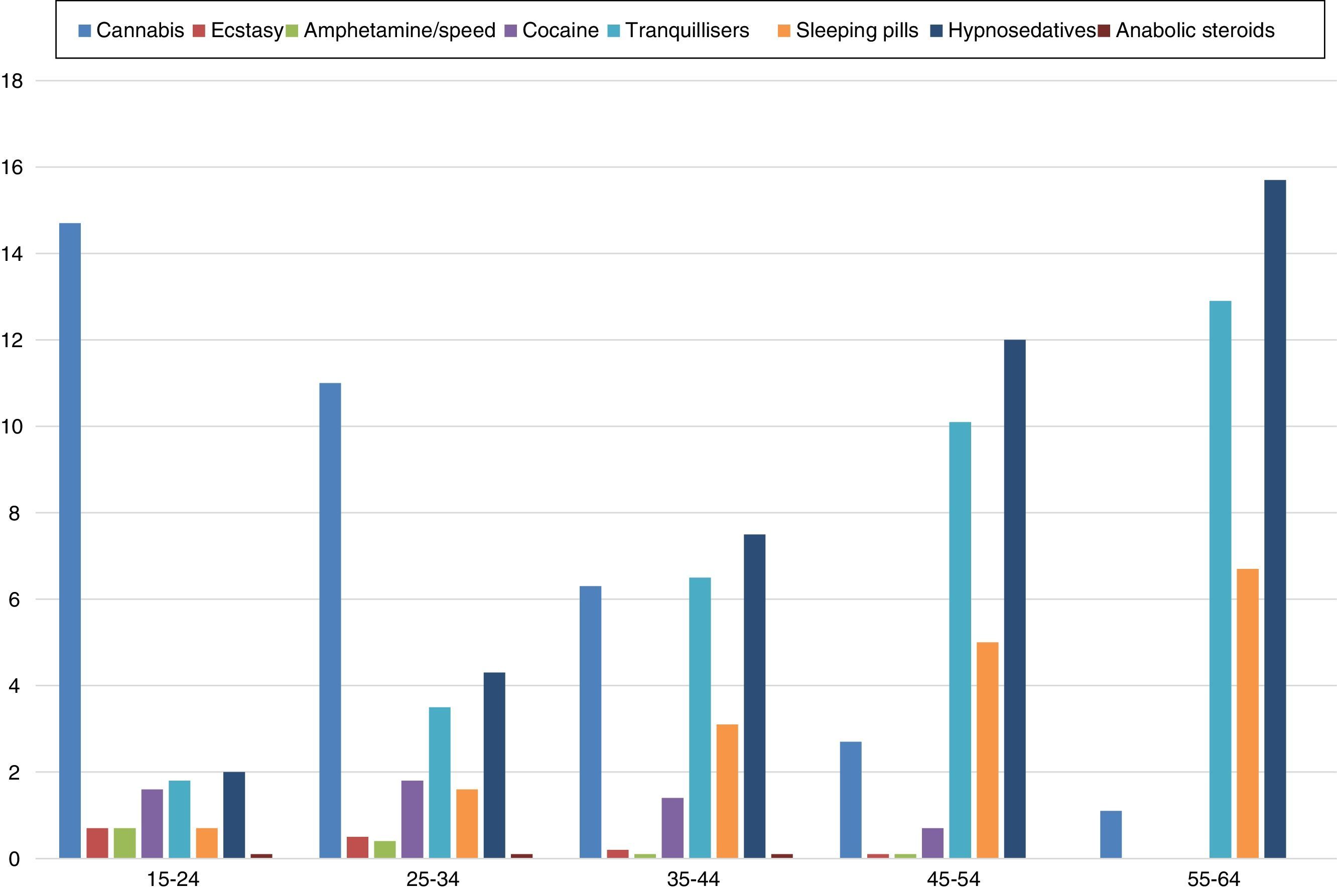
In Spain, alcohol, followed by tobacco, is the most used psychoactive substance in the general population, insofar as 93% of the population has had an alcoholic drink at some point in their lives.5 As far as illegal drugs are concerned, as in Europe overall, cannabis is the most used illegal substance in Spain, followed by cocaine. A minority prevalence below 5% is recorded for ecstasy, amphetamines and hallucinogens, while the use of heroine shows a residual spread in the population.5 In recent years, a slight increase in the use of legal substances (alcohol, tobacco and hypnosedatives) has been observed, while, conversely, the use of illegal drugs has decreased.5Fig. 1 shows the recent use data for these substances in relation to age in Spain in 2013.6
Rates of recent use of various toxic substances in Spain. Source: Observatorio Español de la Droga y las Toxicomanías [Spanish Observatory for Drugs and Drug Addiction].6
Epidemiological studies have shown that the moderate consumption of alcohol is associated with a reduced risk of cardiovascular diseases, representing a protective effect against SCD.7 This reduction has been attributed to its beneficial effects on lipids and high-density lipoproteins (HDL-C), haemostasis and oxidative stress.8 However, alcohol abuse is associated with complex metabolic changes, including multiple alterations which increase the risk of onset of cardiometabolic diseases and atrial fibrillation.8,9
Population studies have shown a clear association between excessive alcohol intake and risk of SCD. The pro-arrhythmogenic effect of alcohol could be responsible for some of these deaths, although the mechanisms are still not properly understood. Some studies have argued that the association could be explained by the finding of a prolonged QT interval in alcoholics.10 It has recently been suggested that alcohol intoxication is a little recognised precipitating factor of Brugada syndrome.11 It has also been hypothesised that the toxic effects of ethanol in the cardiac sodium channels could explain supraventricular and ventricular arrhythmias.12
Furthermore, chronic and excessive alcohol consumption is one of the main causes of dilated cardiomyopathy. Diagnosis is based on the identification of dilated cardiomyopathy, without a justifying cause, in a patient with a history of alcohol abuse. The aetiopathogenic mechanism has not yet been clarified and several hypotheses have been proposed: direct toxic effect on cardiomyocytes, structural changes in contractile proteins, dysfunction of intracellular organelles, abnormalities in calcium homeostasis and direct stimulation of neurohormonal systems. From an anatomopathological point of view, it is indistinguishable from other types of dilated cardiomyopathy.13 Abstinence, and even reduction to moderate alcohol consumption, can result in partial regression of the damage and recovery of the ejection fraction as well as an improvement in functional classification. This is why early diagnosis is of particular interest.14
TobaccoSmoking is a major cause of cardiovascular morbidity and mortality. Deaths from coronary artery disease are significantly reduced in smokers who quit compared to those who continue smoking.15 Specifically, smoking is a major risk factor for acute coronary thrombosis, which causes myocardial infarction and SCD.16 In fact, most SCDs due to coronary thrombosis occur in tobacco smokers.17 Clinical studies have demonstrated that exposure to tobacco facilitates thrombosis by altering the function of endothelial cells, platelets, fibrinogen and coagulation factors. This creates an imbalance between antithrombotic and prothrombotic factors and between pro-fibrinolytic/anti-fibrinolytic factors which facilitates the initiation and spread of thrombosis.18
In forensic studies, in which it can be difficult to obtain detailed information on this history, the histopathological finding of respiratory bronchiolitis can be useful to confirm tobacco use.
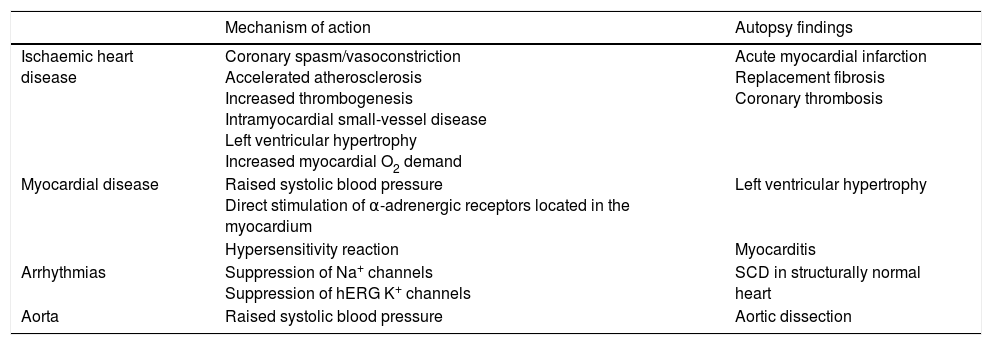
CocaineCocaine use causes significant adverse effects on the cardiovascular system due to its action on the coronary arteries and the myocardium.1,2,19–25 Furthermore, it exerts pro-arrhythmic and significant sympathomimetic effects (Table 1). All of the above means that cocaine is a major risk factor for SCD; a risk that increases when it is used together with other substances, which is especially common in the case of alcohol and tobacco.
Mechanisms associated with the increased risk of sudden cardiac death due to cocaine use.
| Mechanism of action | Autopsy findings | |
|---|---|---|
| Ischaemic heart disease | Coronary spasm/vasoconstriction Accelerated atherosclerosis Increased thrombogenesis Intramyocardial small-vessel disease Left ventricular hypertrophy Increased myocardial O2 demand | Acute myocardial infarction Replacement fibrosis Coronary thrombosis |
| Myocardial disease | Raised systolic blood pressure Direct stimulation of α-adrenergic receptors located in the myocardium | Left ventricular hypertrophy |
| Hypersensitivity reaction | Myocarditis | |
| Arrhythmias | Suppression of Na+ channels Suppression of hERG K+ channels | SCD in structurally normal heart |
| Aorta | Raised systolic blood pressure | Aortic dissection |
SCD: sudden cardiac death.
The concomitant use of cocaine and ethanol elicits the production of an active metabolite, cocaethylene, which is more toxic than cocaine and alcohol when they are used alone and increases the risk of SCD by 25-fold.20 Cocaethylene slows down cardiac conduction and delays repolarisation due to inhibition of the Na+ and K+ channels. The association of cocaine and tobacco facilitates the onset of premature and accelerated coronary atherosclerosis.21
Accelerated atherosclerosis. Repeated administration of cocaine causes endothelial damage which promotes the early onset of coronary atherosclerosis. The study by Lucena et al.1 revealed severe coronary atherosclerosis in 76% of cocaine-related SCDs, with atherosclerosis being the main cause of death in 28%. Cocaine is also associated with intravascular thrombosis in the coronary and peripheral arteries. Scientific evidence suggests that cocaine activates platelet aggregation and stimulates the secretion of thrombogenic substances from the vascular endothelium. Coronary atherosclerosis, with or without occlusive thrombosis, elicits acute or chronic myocardial ischaemia.1,19,22
Intramyocardial small-vessel disease. Myocardial perfusion can reduce despite the epicardial arteries being free from atherosclerosis. This phenomenon is related to the thickening of the middle muscle layer of the intramyocardial arterioles secondary to maintained vasoconstriction caused by the chronic effects of cocaine.1,19,22
Cardiomegaly with left ventricular hypertrophy. Cardiac hypertrophy, on its own and in isolation, increases the risk of SCD.16 The most probable mechanism is the rise in blood pressure which occurs after using cocaine. Another possible mechanism is the direct stimulation of α-adrenergic receptors by cocaine.
Myocardial fibrosis. The high prevalence of myocardial fibrosis in deaths related to cocaine or amphetamines reveals the existence of previous ischaemic episodes. Underlying silent ischaemia, myocardial fibrosis (as a substrate for the re-entry arrhythmic phenomena) or both can increase the risk of arrhythmias. In cases of cardiac hypertrophy secondary to the increased filling pressure, there is also a progressive accumulation of collagen fibres in the myocardial interstitium, especially in the left ventricle. This histological pattern has been called “cocaine-related cardiomyopathy”.24
Eosinophilic myocarditis. This condition has been described as a consequence of the adverse reaction to different drugs, including cocaine.23 The frequency of its onset varies greatly depending on the published studies, ranging from 5%1 to 20%.23
Aortic dissection. Published cases on aortic dissection associated with cocaine are rare and most are related to the use of crack cocaine and to severe hypertension and direct damage on the aorta by catecholamines.25 Most are DeBakey type I dissections and occasionally a cystic degeneration pattern has been found, such as in Marfan syndrome.26
AmphetaminesAmphetamines are substances which are structurally related to phenylethylamine. They include recreational drugs, such as methamphetamine and methylenedioxymethamphetamine, and medicines such as methylphenidate and phendimetrazine. These medicines are currently limited to the treatment of attention deficit hyperactivity disorder, narcolepsy and short-term weight loss.
The pharmacodynamics of amphetamines and their derivatives on the cardiovascular system is similar to that of cocaine, although they present certain particularities. The main toxic effects are mediated by the release of catecholamines, which determines the stimulation of peripheral alpha- and beta-adrenergic receptors, causing a sympathomimetic syndrome. Other pharmacological actions are the inhibition of monoamine oxidase; serotonergic agonist activity; and the increased release of vasopressin.27
Cardiovascular complications are similar to those of cocaine: greater-than-expected heart weight, areas of fibrosis, cardiomyocyte hypertrophy and contraction band necrosis.27,28 The histology of cardiomyopathy associated with methamphetamine is characterised by concentric left ventricular hypertrophy, atypical nuclei, interstitial and perivascular fibrosis, vacuolation of the cardiomyocytes and hypertrophy of the middle layer of small intramyocardial vessels.29
They also present greater susceptibility to accelerated atherosclerosis and to intramyocardial small-vessel disease.29 In the same way as cocaine, vasospasm combined with hypertension and tachycardia can cause reduced cardiac perfusion, even in the absence of atherosclerosis of the epicardial coronary arteries.30
The anatomical changes referred to act as a morphological substrate of SCD and the high concentrations of catecholamines as an initiator phenomenon in the production of arrhythmias.
Some authors have described symptoms of acute myocardial ischaemia after consumption of amphetamine derivatives used in the treatment of attention deficit hyperactivity disorder in adolescents with normal coronary arteries. The symptoms have been related to coronary spasm.31 The chronic use of phendimetrazine in young or middle-aged women has been associated with cardiovascular disease: dilated cardiomyopathy,32 acute coronary syndrome33 and SCD.34 These complications can occur with levels of phendimetrazine in a therapeutic or sub-therapeutic range, and occasionally the patients have taken other psychotropic drugs with an arrhythmogenic effect.
CannabisThe risk of cardiovascular complications related to the use of cannabis is considered low in healthy subjects. By contrast, the acute effects of cannabis can act as precipitating factors of SCD in patients with a high risk of coronary events. It has been reported that the acute use of cannabis increases the risk of infarction almost fivefold, and it is also a significant risk factor associated with the onset of acute cerebral ischaemia.35 In this regard, several cases of SCD associated with the acute use of cannabis or synthetic cannabinoids have been reported.36,37 These findings could be related to the acute effect of cannabis increasing the heart rhythm, and in some cases also increasing blood pressure. Likewise, some data indicate that Δ9-tetrahydrocannabinol, the main psychoactive ingredient of cannabis, could also induce vasoconstriction. These effects could be much more intense if synthetic cannabinoids or varieties of cannabis with a high Δ9-tetrahydrocannabinol content are used.
OpioidsThe cardiovascular effects produced by heroin do not seem to play an important role in the morbidity and mortality of this drug. Death is generally due to respiratory depression or to an adverse drug reaction, and is not usually associated with SCD.
Methadone is the drug most commonly used as opioid replacement therapy in addiction therapies, but it is also used as an analgesic and as a recreational drug. Its adverse effects include respiratory depression and QT prolongation. It is therefore recommended to perform a prior ECG on opioid addicts who are integrated into methadone programmes.38 A link between methadone, at non-toxic levels, and SCD in a structurally normal heart has been reported in forensic studies.39 These deaths are related to an arrhythmic mechanism, as one of methadone's side effects is the production of acquired long QT syndrome with prolonged ventricular repolarisation and an increase in the risk of onset of “torsades de pointes”.
Performance-enhancing drugsThe use of substances with the aim of illegally enhancing sporting performance is recognised as common practice and extends beyond elite sporting activities, with a cost for health and a link to SCD which is becomingly increasingly well-known.40 The World Anti-Doping Agency annually updates its list of “prohibited” substances.41 The list includes anabolic-androgenic steroids, peptide hormones, β2-agonists, diuretics such as “masking agents”, stimulants, narcotics, cannabinoids, glucocorticosteroids, alcohol and beta-blockers. Almost all of these illegal substances, especially anabolic steroids, stimulants and peptide hormones, can cause arrhythmogenic effects both due to an increase in focal automatism and to enhancing electrophysiological heterogeneity, and they are triggering factors of re-entry arrhythmias. All of this occurs both at the supraventricular and ventricular level, with the known vital risk that it entails, particularly in the context of facilitating elite sporting performance. The mechanisms of intervention are very diverse. Some are not fully established and almost always have a link to the pattern of use and individual susceptibility.
Anabolic-androgenic steroids deserve special mention because of their particularly deleterious cardiovascular consequences. They are usually consumed together with “steroid accessory substances” for various kinetic and dynamic synergy reasons, such as growth hormone, insulin or diuretics.42,43 Due to their anabolic effect on the metabolism of nitrogen, they cause muscle hypertrophy. In the myocardium, it translates into the abnormal apposition of muscle fibres and left ventricular hypertrophy, as well as into diastolic dysfunction,44 and in the long term to subendocardial ischaemia, cardiomyocyte disorganisation with myocardial fibrosis and dilation and systolic dysfunction. These changes are not explained by the adaptation of the heart to exercise (“athlete's heart”).45 In the initial phases, these changes are partially reversible.46
Furthermore, these substances have collateral systemic effects: they alter the lipid metabolism and are linked to endothelial dysfunction, hypertension,47 procoagulability and therefore coronary artery disease. They alter the parasympathetic innervation, cause QT interval dispersion and increase intracellular calcium,48 which is a potential cause of arrhythmias and SCD when associated with the histological abnormalities mentioned. These effects are dose-dependent and are almost always related to the quantity and time of use.
Therefore, the clinical diagnosis of a potentially fatal cardiac arrhythmia, particularly in the presence of a latent electrophysiological substrate, which includes some hereditary cardiomyopathies with a risk of sudden death, should raise the suspicion that an illegal substance could be a precipitating cause, and this link should be investigated.
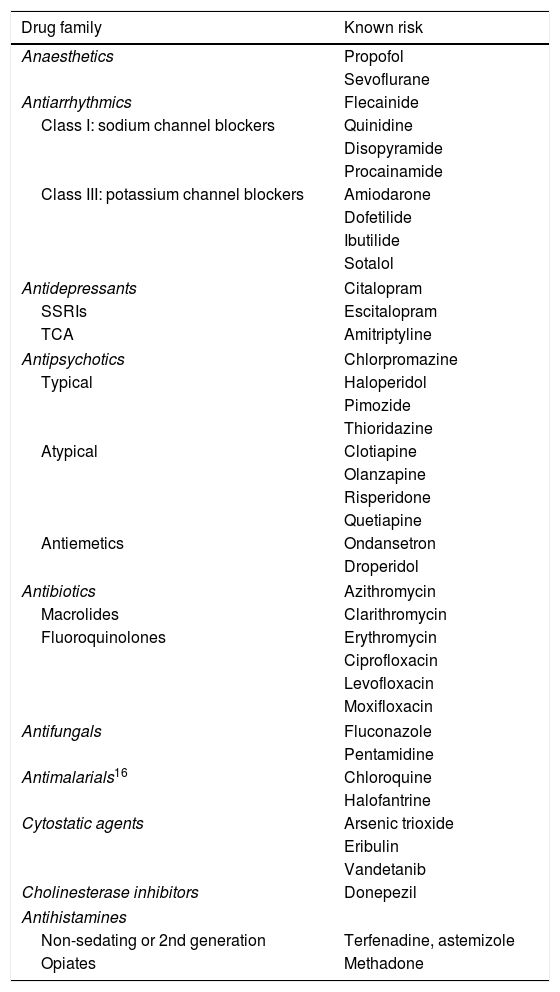
Acquired long QT syndrome and drug-induced proarrhythmiaAcquired long QT syndrome is a condition which is related to SCD in a normally structured heart and is caused mainly by the use of drugs.49 The capacity to reduce cardiac excitability (with an electrocardiographic translation of QT interval prolongation) is a shared property of anti-arrhythmic drugs and those with a non-cardiac therapeutic target. The monitoring centre of the World Health Organisation50 issues reports regarding drugs with the capacity to induce symptomatic long QT syndrome (Table 2). Alongside this, the Mayo Clinic has contributed to structuring this alert and has developed a risk scale, which is widely used in daily clinical practice and on which the updated list on the CredibleMeds Website is based.
Drugs with known risk of torsades de pointes.
| Drug family | Known risk |
|---|---|
| Anaesthetics | Propofol |
| Sevoflurane | |
| Antiarrhythmics | Flecainide |
| Class I: sodium channel blockers | Quinidine |
| Disopyramide | |
| Procainamide | |
| Class III: potassium channel blockers | Amiodarone |
| Dofetilide | |
| Ibutilide | |
| Sotalol | |
| Antidepressants | Citalopram |
| SSRIs | Escitalopram |
| TCA | Amitriptyline |
| Antipsychotics | Chlorpromazine |
| Typical | Haloperidol |
| Pimozide | |
| Thioridazine | |
| Atypical | Clotiapine |
| Olanzapine | |
| Risperidone | |
| Quetiapine | |
| Antiemetics | Ondansetron |
| Droperidol | |
| Antibiotics | Azithromycin |
| Macrolides | Clarithromycin |
| Fluoroquinolones | Erythromycin |
| Ciprofloxacin | |
| Levofloxacin | |
| Moxifloxacin | |
| Antifungals | Fluconazole |
| Pentamidine | |
| Antimalarials16 | Chloroquine |
| Halofantrine | |
| Cytostatic agents | Arsenic trioxide |
| Eribulin | |
| Vandetanib | |
| Cholinesterase inhibitors | Donepezil |
| Antihistamines | |
| Non-sedating or 2nd generation | Terfenadine, astemizole |
| Opiates | Methadone |
SSRIs: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressant.
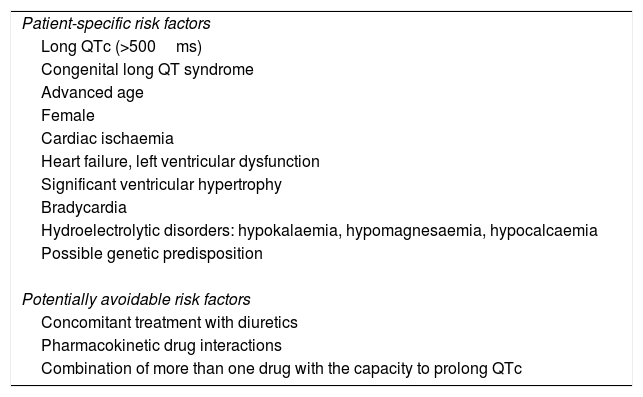
The QT interval depends on multiple ion channels, and it has been recognised as a risk marker for ventricular fibrillation in the form of torsades de pointes associated with the effect of different drugs.51 This occurs at conventional doses, even sometimes at trace plasma levels, without homogeneous behaviour over time and an ambiguous class effect within each drug family.49–52 This makes it extremely complicated to predict the real risk to which each patient is exposed, as it is determined by multiple acquired and innate factors associated with a special susceptibility (Table 3).3,52
Risk factors for drug-induced arrhythmogenicity.
| Patient-specific risk factors |
| Long QTc (>500ms) |
| Congenital long QT syndrome |
| Advanced age |
| Female |
| Cardiac ischaemia |
| Heart failure, left ventricular dysfunction |
| Significant ventricular hypertrophy |
| Bradycardia |
| Hydroelectrolytic disorders: hypokalaemia, hypomagnesaemia, hypocalcaemia |
| Possible genetic predisposition |
| Potentially avoidable risk factors |
| Concomitant treatment with diuretics |
| Pharmacokinetic drug interactions |
| Combination of more than one drug with the capacity to prolong QTc |
Another syndrome which has been linked to SCD in structurally normal hearts is Brugada syndrome. There are several drugs (which inhibit the sodium channel SCN5, among others) which produce an electrocardiographic pattern typical of Brugada and are therefore associated with an arrhythmic risk. There are a series of medicines which should be avoided in patients diagnosed with this syndrome due to their degree of risk. There are clinical practice guidelines which also establish risk gradients (www.brugadadrugs.org).53
The prevention of arrhythmic SD associated with the pro-arrhythmogenic effects of drugs may require hospital monitoring, minimising synergistic interactions as much as possible and, above all, preventing exposure, favouring the selection of drugs with a better safety profile, particularly in cases with greater susceptibility.54,55
There is also evidence concerning the adverse cardiovascular risk profile for antipsychotic drugs, especially phenothiazines, related to an increase in classic cardiovascular risk factors.56 The correlation is difficult to establish due to the presence of multiple confounding factors in patients with schizophrenia or bipolar syndrome: increased consumption of tobacco and alcohol, and a greater tendency towards being obese and physically inactive. However, several studies have shown a trend towards the development of insulin resistance and dyslipidaemia, as well as reduced distensibility in medium-calibre arteries in psychiatric patients undergoing antipsychotic treatment compared to controls with no treatment.57
In the forensic pathology field, there is also evidence that some psychotropic drugs increase the risk of SCD, even at post-mortem therapeutic concentrations. In a study on forensic autopsies, it was observed that the percentage of cases with positive toxicology (psychotropic drugs were the most frequently detected substances) was significantly higher in SCD in structurally normal hearts than in SCD with structural pathology, which suggests that these substances may play a proarrhythmic role.3 The possibility of the intervention of these proarrhythmic drugs should be assessed in SCD in normally structured hearts.
Implications for forensic pathologyIn the event of SCD, a complete forensic investigation should be conducted to establish the cause of death and its triggering factors, including toxic substances, even when structural disease which justifies the death has been detected in the autopsy.58 A correct interpretation on the cause and mechanism of death should be established based on clinical, histopathological and toxicological criteria.
The macroscopic and histopathological study will also make it possible to determine the existence of organic disease in relation to the acute or chronic use of toxic substances. Toxicological analyses are essential, not only to exclude death of toxic origin, but also to determine the use of drugs, performance-enhancing drugs and medicines which may have been able to act as triggering factors of the death.
In terms of sampling, it is essential to send blood (ideally peripheral blood), urine and vitreous humour in all cases. Depending on the case, other samples, such as gastric content, could be sent. Analysis of hair is useful to establish the history of exposure to recreational drugs and to determine the implication of a substance in the cause of death. It must be remembered that some modern synthetic drugs, such as synthetic cannabinoids, are not currently detected in the routine screening of toxicology laboratories.
Although the association between drugs and SCD is well established, in practice it may be difficult to determine the exact underlying mechanism in a specific case; i.e., to determine what contribution the cardiac structural disease has played, what role the toxic substance has played and what synergistic effect there has been between both elements. This is due to the interplay of multiple factors, such as the route of administration, the dose used, tolerance, individual vulnerability, the associated risk factors, the concomitant use of diverse substances which interact with them, etc. Interpretation is further hindered by the complexity of post-mortem toxicology and the overlap which exists between therapeutic and toxic concentrations for many substances.
The possibility of using recreational drugs should be taken into account in all sudden deaths of adolescents, young people or even middle-aged adults, particularly males. It would also be advisable to rule out the use of performance-enhancing drugs in athletes and to assess the influence of performance-enhancing drugs in patients who took psychotropic drugs, even with post-mortem therapeutic levels. In the absence of another cause of death, the possibility of arrhythmic death due to acquired long QT syndrome or Brugada syndrome should be considered.
In the prevention of SCD, it is important to identify the modifiable risk factors which trigger fatal arrhythmia, as this offers a good approximation for the development of effective strategies. The recent frequency of consumption of alcohol and/or recreational drugs is relatively high. Therefore, actions aimed at detecting and controlling these factors and educational campaigns on the cardiovascular risks of recreational drugs could be effective at preventing SCD.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morentin B, Callado LF, García-Hernández S, Bodegas A, Lucena J. Papel de las sustancias tóxicas en la muerte súbita cardiaca. Rev Esp Med Legal. 2018;44:13–21.





![Rates of recent use of various toxic substances in Spain. Source: Observatorio Español de la Droga y las Toxicomanías [Spanish Observatory for Drugs and Drug Addiction].6 Rates of recent use of various toxic substances in Spain. Source: Observatorio Español de la Droga y las Toxicomanías [Spanish Observatory for Drugs and Drug Addiction].6](https://static.elsevier.es/multimedia/24454249/0000004400000001/v1_201802240429/S2445424918300037/v1_201802240429/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)