Advanced parental age (APA) has been associated with an increased risk for autism in the offspring. One explanatory model includes delayed fatherhood in parents with autistic traits.
Material and methodsWe investigated (1) whether autistic traits in parents, evaluated with the Autism-spectrum Quotient (AQ), correlate with APA in 128 families, (2) in 83 trios with genetic data available, whether AQ correlated with polygenic vulnerability calculated by Polygenic Risk Scores (PRS). We stratified the analyses by as per DSM-IV autism subtypes and by parental sex.
ResultsWe found a statistically significant relation between AQ and APA (r=0.207, p=8.39×10−4, n=256), significantly only in mothers (r=0.233, p=8.23×10−3, n=128) and in Asperger Syndrome (AS) (r=0.319, p=0.034, n=44). There was a significant association between PRS and AQ in the mothers of the participants with AS (β (95%CI)=3.28 (0.03–6.59); p=0.047).
ConclusionsThese results show that, in this sample, older mothers present more autistic traits, and APA seems to relate to an AS profile. Furthermore, PRS is significantly associated with maternal AQ of AS subjects. Consequently, a higher polygenic maternal contribution (both by AQ and PRS) seems to contribute to an AS profile.
Autism Spectrum Disorders (ASD) conform a group of neurodevelopmental disorders with an estimated prevalence between 8 and 17 cases per 1000 in the general population.1–3 Researchers have described a multifactorial etiology that may combine genetic and environmental factors. Some recent reviews emphasize the importance of how non-genetic factors interact with genetic factors, increasing the risk of autism.4,5 Among non-genetic factors, advanced parental age at conception (APA) has been associated with increased risk for autism and other neurodevelopmental disorders in offspring.4,6,7–10 These studies have mainly reported that older fathers have increased risk of having children affected by ASD. Researchers have proposed that de novo mutations in male germ cells accumulated during aging increase the risk of neurodevelopmental disorders as schizophrenia or autism.11,12 However, de novo mutations only account for a part of the risk associated with APA,13 and other researchers have proposed alternative (but not mutually exclusive) explanatory models, including the possibility that delayed fatherhood is a consequence of autistic traits in the progenitor.14 Delayed motherhood has also been associated with an increased risk of ASD in the offspring,15 particularly in high-functioning subjects,16,17 with no data on the possible mechanisms.
Autistic traits seem to be continuously distributed in the general population, according to studies that have administered autistic-traits questionnaires to non-clinical population (such as the Autism-Spectrum Quotient for adults (AQ).18 Autistic traits have also been evaluated in specific populations18,19; for example, AQ has been used to compare parents of ASD subjects with parents of typically developing children in China, Istanbul, Iran, Australia and other countries.20–23 Parents of ASD participants in those studies showed higher AQ scores than parents of control participants.
Gratten et al.14 suggested that autism traits in parents would be associated with an increased common variation risk for autism and subsequently with higher autism polygenic risk scores (PRS). To our knowledge, that association has not yet been explored. The latest ASD Genome Wide Association Studies (GWAS)24 demonstrated that the polygenic contribution in Asperger Syndrome (AS) is almost double that of other subtypes of ASD. Therefore, patients with AS might be the most informative ASD population for exploring the relationship between AQ and PRS and delayed parenthood.
The main objectives of this study were to examine whether autistic traits in parents of ASD subjects, measured via the Autism Spectrum Quotient (AQ), correlate with parental age at conception, and dissect to what extent this correlation differs according to ASD subtype diagnosis. We also explored the relationship between autistic traits in the parents and polygenic vulnerability. Finally, we surveyed the relationship between autistic traits in mothers and fathers.
We hypothesized that (i) advanced parental age at the time of conception would correlate with parental autism traits (AQ scores), particularly in fathers of subjects with AS, and (ii) polygenic contribution in parents would correlate with AQ measures.
MethodsParticipantsThe recruitment of subjects with ASD took part at a specialized outpatient clinic, AMITEA (Comprehensive Medical Care-Autism Spectrum Disorders),25 at the Child and Adolescent Department of Psychiatry, Hospital General Universitario Gregorio Marañón, Madrid, Spain. The inclusion criteria for this study were: at least 3 years of age, Spanish as a first language, and a diagnosis of ASD in the offspring. The subjects were included in the “Study of differential pathophysiological pathways associated with distinctive phenotypes in a sample of 200 patients with Autism Spectrum Disorder” funded by F.I.S. Carlos III, Ministry of Economy and Competitiveness PI14/02103 and the samples stored at the Ministry of Health Collection of ASD (sample # C.000148). The Hospital's Clinical Research Ethics Committee approved procedures and ASD subjects and their parents or legal guardians gave written informed consent. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Demographic and clinical dataThe diagnosis was given by Child psychiatrists with extensive experience in ASD and research training in the Autism Diagnostic Observation Schedule (ADOS),26 following standard procedures.27 The procedure included taking developmental, medical and psychiatric histories, review of previous reports, and non-structured observation of the child. When necessary, the ADOS and/or the Autism Diagnostic Interview – Revised (ADI-R)28 were administered. Finally, clinical judgment taking into account all the above information and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision and Fifth Edition (DSM-IV-TR and/or DSM-5)29 criteria were applied. In order to check if autistic traits were differentially present in parents of subjects with different subtypes of ASD, we divided the sample according to DSM-IV-TR in Asperger Syndrome (AS), Autistic Disorder (AUT) and Pervasive Developmental Disorder, Not Otherwise Specified (PDD-NOS).
For descriptive purposes we collected any available evaluations of the patients that reflected cognitive performance, including Intelligent Quotient (IQ) or cognitive development index in cases without language or early age. Since those instruments have a mean of 100 and a standard deviation 15 reference values, for our purpose, the results of these instruments were combined in a single index, which we called cognitive index. Cognitive performance and autistic severity were not collected as part of this study. In a subsample of the patients included for current analyses this information was available. We report this data only for descriptive purposes.
Demographic data from ASD subjects and their parents (age, sex, ethnicity, age at conception) and the ASD-subjects’ early clinical presentation (developmental regression, age of recognition of first autism symptoms and language impairment) were collected using a self-devised structured interview with both parents. Previous studies have used age range categories to define parental age, following the ones exposed by Lundström in 201030: <25, 25–34, 35–44, 45–50, and >51 years. Other authors define advanced parental age as 10 years older than average.14 Given the lack of available consensus in the literature with regards to a cut-off point that categorizes parental age as advanced or not and the nature of the variable per se, in this study parental age at conception was measured as a continuous variable.
Parental autism traitsParental autism traits were evaluated with the Spanish version of the autism-spectrum quotient for adults (AQ).18 The AQ is a self-report questionnaire consisting of 50 statements, with 4 possible answers each. The results are presented as a total score, which quantifies the level of autism traits (in our case in parents of ASD subjects).
Polygenic risk scoresFor the polygenic risk scores (PRS), we used exome data from 242 ASD trios from Madrid (Spain) sequenced as part of the Autism Sequencing Consortium (ASC). Exome sequencing was performed at the Broad Institute Genomics Platform using an Illumina HiSeq sequencer. Variant calling was performed at the entire consortium dataset as explained by its main publication.31 Briefly, raw sequencing data were processed using the Genome Analysis Toolkit (GATK)32 to produce variant files in VCF 4.1 format. Genetic variants within. Major Histocompatibility Complex (MHC) were removed. Variant call accuracy was estimated using the GATK Variant Quality Score Recalibration (VQSR) approach. Calls with a depth of coverage less than 10 or greater than 1000 were filtered out.
Exome-based VCF files after QC filtering were then imputed at the Michigan Imputation Server (https://imputationserver.sph.umich.edu/). We used the 1000 Genome Project phase 3 v.5 reference panel in order to capture genomic variants beyond the exome. Then PRS were calculated using this imputed data as the target sample and GWAS summary statistics from the PGC repository (https://www.med.unc.edu/pgc/results-and-downloads) as the discovery sample,24 following previous work.33 Next ASD PRS was assigned to each individual from the target sample as the sum of the number of effect alleles weighted by their effect in the independent discovery sample. Only biallelic variants with an imputation quality score>0.9 and minor allele frequency (MAF)>0.1% were considered. Indels were also excluded. Clumping was performed using PLINK v1.9 code “--clump-r2 0.1 --clump-kb500”. We used Flip Strand to identify and remove ambiguous positions. We generated different PRS using various P thresholds from the discovery sample (p<0.01, 0.05, 0.1, 0.2, 0.5 and 1). In order to select the appropriate P threshold, we calculated PRS from both parents and performed polygenic transmission scores (pTDT), as previously performed in ASD.34 We selected the PRS whose p threshold had the lowest p-value in pTDT (supplementary table). Common variants do account for a great part of ASD liability. However, a large recent GWAS study comprising close to 20,000 patients and 30,000 controls has shown that a PRS that includes variants associated with common ASD-comorbidities to the risk associated with ASD-related variants explains more of the ASD risk that the PRS calculated only with ASD-related variants.35 Therefore, we calculated PRS for other disorders with well-known comorbidity with autism36 in the same target sample (Major Depression (MDD), Attention Deficit and Hyperactivity Disorder (ADHD), Obsessive Compulsive Disorder (OCD), Generalized Anxiety (ANX) and Schizophrenia (SCZ); summary statistics were used). A combined PRS (PRScomb), using ASD and comorbid disorders summary statistics was calculated,37–41 following a previously published model.35 The combinatorial model requires weighing each disorder's relative contribution, so we used an estimated value from transmission t test from parents to offspring, for each disorder separately. Then we constructed the combinatorial PRS model using the estimated values to weight each comorbid disorder's contribution to the PRScomb score. Analyses using ASD PRS instead of PRScomb show similar but no significant results (data not shown).
Maternal and paternal separated and combined PRS were used to study their association with AQ measures in the whole sample and dividing by ASD subtypes. To simplify, we will refer to PRScomb as PRS throughout the results section.
In order to assess the relationship between PRS and AQ measures, linear regression models were performed, using sex and the first 10 multidimensional scaling (MDS) ancestry components as covariates. PLINK 1.9 was used to calculate the 10 MDS ancestry components from exome-imputed data for the 242 ASD trios from Madrid.
Rare de novo variant analysisRare damaging variation across the ASD trios with available exome and parental AQ data (N=83 out of 128) was extracted from sequencing data generated by the Autism Sequencing Consortium (ASC). We have considered protein truncating de novo rare variation (dnPTV) as nonsense, frameshift, or splicing variants at loss-of-function intolerant genes (pLI>0.9) and present at ExAC [http://exac.broadinstitute.org/] or 1000 genomes database [http://www.internationalgenome.org/] at an allelic frequency less than 0.1% in European population. In order to remove likely artifacts, we only considered singleton variants for the analyses.
Statistical analysisWe describe all variables used with the mean and standard deviation for continuous variables and frequencies and percentages for discrete variables.
Normality of distribution of the quantitative variables (parental age, AQ total scores and PRS) was assessed with the Kolmogorov-Smirnov test. These analyses suggested that the AQ total score, parental age and PRS had non normal distributions (p<0.05).
The relationship between autism traits in parents and age at conception was assessed with the Spearman correlation. Linear regression models were used to analyze the contribution from PRS to AQ measures, using sex and MDS ancestry components as covariates. Analyses were performed by subsampling by ASD subtype and considering mothers and fathers separately. Relationship between AQ and de novo genetic variation (dnPTV) was evaluated by logistic regression models using dnPTV as dependent variable (1 for presence/0 for absence of variant) and AQ as independent variable, considering mothers and fathers separately.
For descriptive purposes, we evaluated possible differences in autism traits in parents depending on their sex (using the U Mann–Whitney test) and whether parents were matched according to their autism traits (using the Spearman correlation). Then the Wilcoxon test was used to check if our sample's values were equivalent to the biggest previous European sample in the literature.42 Finally, we compared the distribution of AQ scores in our sample (fathers and mothers separately) with the Wheelwright study's population42 using compliance testing.
Statistical analyses were performed using R v.3.6.0.43 or PASW Statistics 18.0 for Windows,44 with a significance p-value threshold set at <0.05.
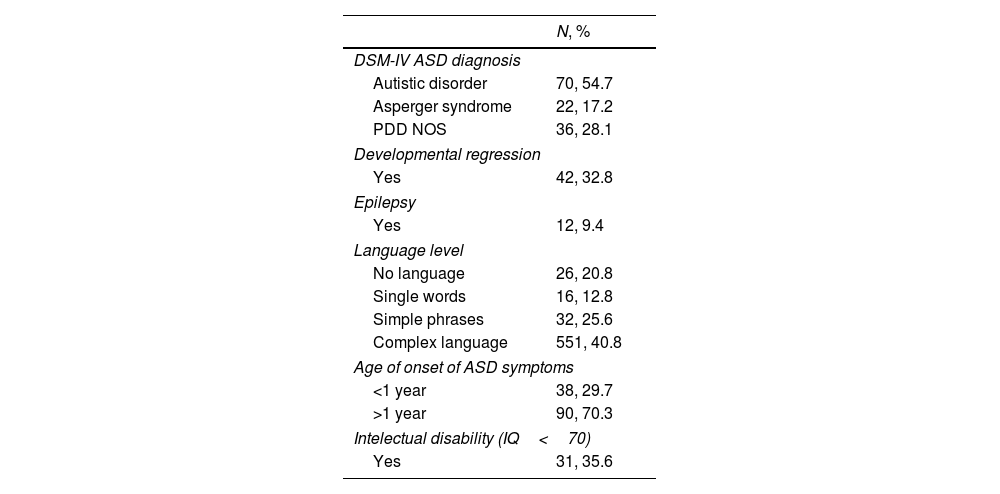
ResultsThis study included 128 subjects with ASD (almost 90% male and Caucasian) between the ages of 3 and 36, mean age 11.85 (SD 7.72) and both of their parents. Full details of the clinical characteristics of the ASD individuals are shown in Table 1.
Clinical characteristics of the sample.
| N, % | |
|---|---|
| DSM-IV ASD diagnosis | |
| Autistic disorder | 70, 54.7 |
| Asperger syndrome | 22, 17.2 |
| PDD NOS | 36, 28.1 |
| Developmental regression | |
| Yes | 42, 32.8 |
| Epilepsy | |
| Yes | 12, 9.4 |
| Language level | |
| No language | 26, 20.8 |
| Single words | 16, 12.8 |
| Simple phrases | 32, 25.6 |
| Complex language | 551, 40.8 |
| Age of onset of ASD symptoms | |
| <1 year | 38, 29.7 |
| >1 year | 90, 70.3 |
| Intelectual disability (IQ<70) | |
| Yes | 31, 35.6 |
PDD NOS: Pervasive Developmental Disorder – Not Otherwise Specified.
Parents of the 128 ASD subjects (n=256) were also evaluated. Mothers’ ages ranged from 22 to 48 years old, mean age 32.62 (SD 4.66) and fathers’ ages from 22 to 58, mean age 34.64 (SD 5.62).
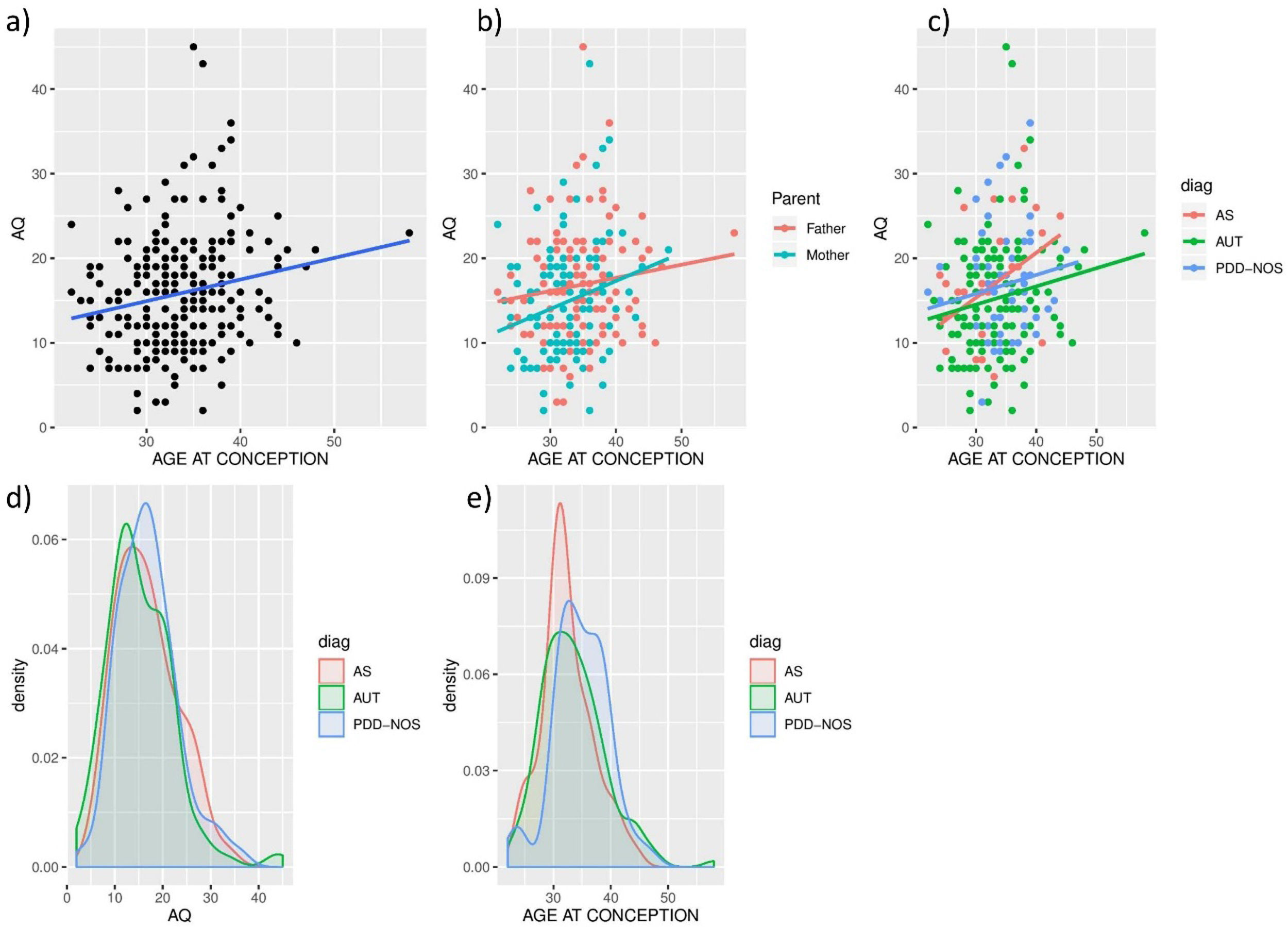
Parental ASD traits and age at conceptionIn this dataset, a statistically significant positive relationship was found between parental age at conception and AQ total scores (rho=0.207, p=8.39×10−4, n=256). Separately, this correlation is present in mothers (rho=0.233, p=8.23×10−3, n=128, but not in fathers (rho=0.123, p=0.166, n=128). See Fig. 1a, b for these results. When analyzing these correlations by different subtypes of autism, significant positive correlations were found in the case of subjects with AS (rho=0.319, p=0.034, N=44) but not in the AUT (rho=0.200, p=0.069, N=131) or PDD-NOS (rho=0.142, p=0.272, N=62) subsamples (Fig. 1c). Given the small sample size we did not proceed to subdivide the correlations with different types of autism by parental sex.
Relationship between parental autistic traits (AQ) and age at conception. Spearman correlation was calculated for the (a) general sample (rho=0.207, p=8.39×10−4*, n=256), (b) divided according to parents’ sex (mothers rho=0.233, p=8.23×10−3*, n=128, fathers rho=0.123, p=0.166, n=128) (c) according to DSM-IV subtypes (in AS rho=0.319, p=0.034*, N=44; in AUT rho=0.200, p=0.069, N=131; in PDD-NOS rho=0.142, p=0.272, N=62). Density plots are shown for (d) AQ values (Kruskall-Wallis test–p>0.05 in any case) and (e) age at conception’ (PDD-NOS – AS Kruskal–Wallis test-p=0.00364; PDD-NOS – ASD Kruskal–Wallis test-p=0.020).
No differences in parental AQ values were found across ASD subtypes (Fig. 1d; KW test – p>0.05 in any case).
Age at conception in parents of subjects with PDD-NOS (age (95%CI)=34.74 (33.76–36.08) is higher than in parents of subjects with AS (Age (95%CI)=32.39 (31.04–33.74); Kruskal–Wallis test-p=0.00364; Fig. 1e) or AUT (Age (95%CI)=33.47 (32.53–34.41); Kruskal–Wallis test-p=0.020; Fig. 1e), with no significant difference between AS and AUT parents’ age (Kruskal–Wallis test-p=0.351). Results in Fig. 1d, e indicate that, in spite of the higher correlation between age at conception and parental AQ observed in AS trios (Fig. 1c), older parents are at higher risk for PDD-NOS than AS or AUT autistic subtypes.
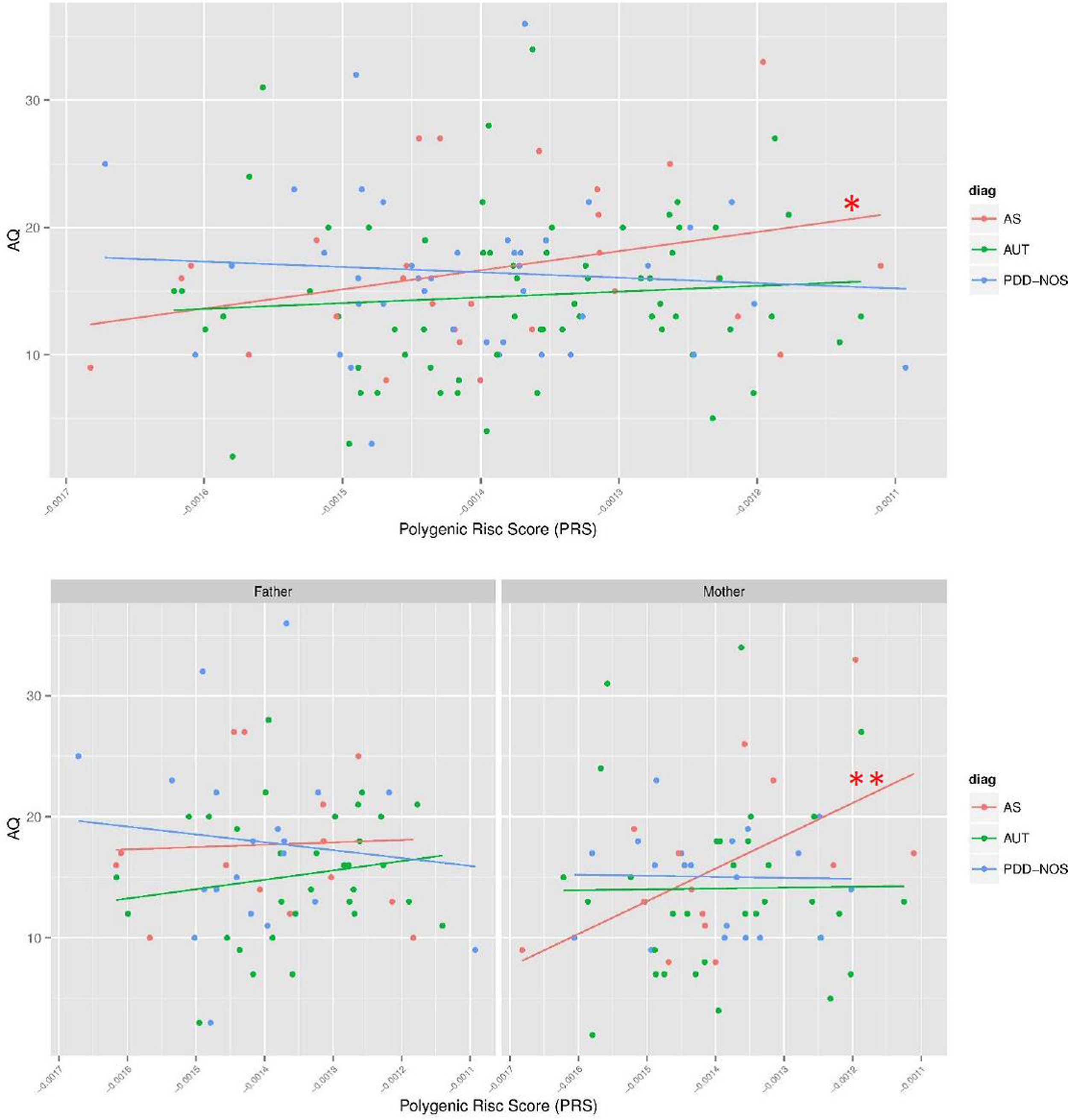
Parental ASD traits and polygenic risk score by autism subtypeWe calculated PRS on those trios with parental AQ and exome information available, (N=83 out of 128). As explained in Methods, PRS calculations were performed using imputed exome data and available summary statistics from ASD, and weighing other comorbid disorders. We used a linear regression model to study whether PRS significantly explained AQ scores in the surveyed DSM-IV ASD subtypes, considering sex and 10 first multidimensional scaling (MDS) ancestry components as covariates in the model.
There is a trend toward significance between parental PRS and AQ measures in the parents of the AS subsample (β (95%CI)=1.9 (−0.29 to 4.08); p=0.086; Fig. 2), but not in the AUT or PDD-NOS subsamples (p>0.1; Fig. 2).
Associations between PRS and AQ measures in parents of ASD trios. Regression models were performed for the three DSM-IV subtypes (AS, AUT and PDD-NOS) and, additionally, for mothers (AS_MOTHERS) and fathers (AS_FATHERS) of AS trios. To facilitate comparison of effects, the PRS were standardized. * p<0.1; ** p<0.05.
As the relationship between age at conception and AQ differed for mothers and fathers of AS trios, we also divided the PRS analysis in both parents independently for AS subtype. Higher PRS was associated with increased AQ across mothers of subjects with AS (β (95%CI)=3.28 (0.03–6.59); p=0.047), but not across fathers of these subjects (β (95%CI)=0.67 (−3.34 to 4.68); p=0.701; Fig. 2).
We have also evaluated the relationship between parental AQ and presence of dnPTV across the affected children. No significance was observed when evaluating the effect of probands dnPTV on parental AQ, either across mothers (β (95%CI)=−0.012 (−0.102 to 0.077); p=0.790) or fathers (β (95%CI)=−0.043 (−0.138 to 0.052); p=0.377).
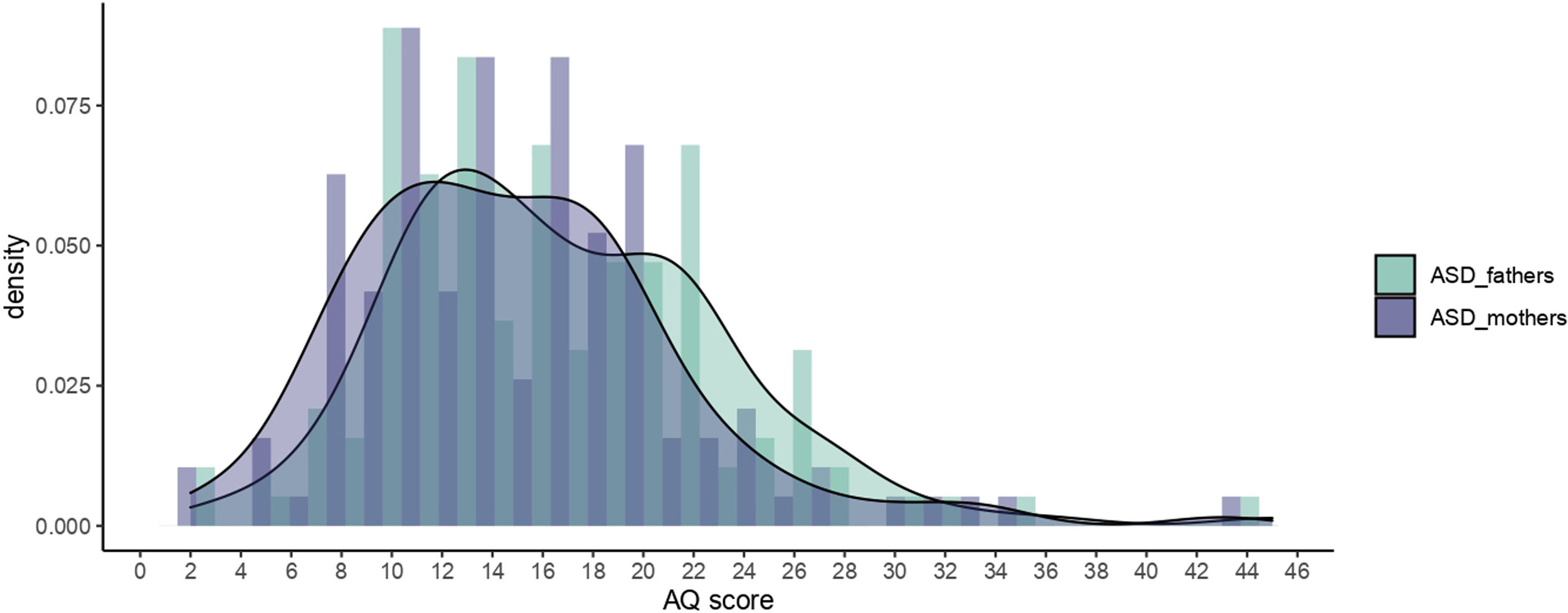
ASD traits in the parents of ASD subjectsFathers had significantly higher AQ scores than mothers (U=6759.5 p=0.009), with AQ mean score being 16.79 SD=6.56 (range 3–45) and 14.84 SD=6.5 (range 2–43) for fathers and mothers, respectively. There was a significant positive modest relationship between AQ scores of both parents r=.234 p=0.008.
Given that there are no Spanish data on the distribution of ASD traits (measured with the AQ) among parents of individuals we autism, we decided to compare our results with those from the original British sample. Therefore, we compared the distribution and mean values of ASD parents from our sample (more than 80% of Spanish origin) with those from the Wheelwright et al. sample of the British population42 (see Fig. 3). In that sample 41.5% of the offspring were reported to have autism, 41.38% Asperger syndrome (AS), 10.56% high functioning autism (HFA) and 6.56% pervasive development disorder (in our sample these percentages were 55% autism, 15.6% AS and 29% pervasive development disorder). Our sample had lower mean values in AQ test than Wheelwright et al.: mothers mean=16.4 vs 14.84 (95%CI) (13.73–16.01) and fathers mean=19.2 vs 16.79 (95%CI) (15.71–18) (mothers Z=−3.202 p=0.001, fathers Z=−4.466 p<.001) so we rejected the assumption that our population is equivalent to Wheelwright et al. In fact, we noted that Wheelwright et al.’s range of scores exceeded our maximum scores (Fig. 3).
DiscussionWe show that, within a sample of parents of ASD subjects, there is a direct and significant relationship between autistic traits (as measured with the AQ questionnaire) and age at conception. This supports our main hypothesis. However, the relationship is dependent on sex, as mothers, but not fathers, account for the significant part of the relationship. Moreover, our results show differential correlations when the proband sample is divided into DSM-IV subtypes: in our sample, there is only a significant relationship between AQ and parental age of AS subjects.
Interestingly, we found that fathers showed more autistic traits than mothers, and that PDD-NOS subjects were conceived at an older parental age.
Although our data need replication and may not be robust enough due to a small sample size, our results support the hypothesis that advanced parental age could be a consequence of autistic traits in the progenitors,14 at least for mothers.
There is consistent evidence of biological and genetic overlap between ASD and autistic traits in the general population45 with higher estimates of heritability for autistic traits than for ASD per se.46 AS has been described as a distinct biological subtype of ASD based on the latest GWAS to date, with higher contribution from common genetic variation.35 Our results here suggest an impact of maternal AQ in ASD risk which is restricted to AS trios. Therefore, our data support the hypothesis that mothers with more autistic traits do conceive offspring more frequently diagnosed with AS.
PRS estimations have been proven to be useful in analyzing the contribution of common genetic variation from a particular disease or trait.47–49 In our dataset, a combined-PRS using ASD and comorbid disorders’ contributions33 significantly predicted maternal, but not paternal, AQ in AS subjects, while de novo variation does not. This result could be explained, in part, by the polygenic overlap between AS and related psychiatric conditions.50,51 The results do not retain significance after multiple testing correction but do converge with the results describing the relationship between AQ and age at conception. We strongly encourage further studies aiming to replicate these findings to ensure a larger sample size.
In regard to the higher polygenic maternal contribution to the risk of AS in offspring described here (both by AQ and PRS analyses), a recent study reported that maternal PRS contributes to neurodevelopmental disorders in offspring by increasing the risk for exposure to certain adverse environments during pregnancy.52 As an early neurodevelopmental disorder,53 ASD outcomes in offspring may be more affected by maternal than paternal environment.54
ASD subtypes disappeared from recent versions of psychiatric classifications (DSM-5 and ICD-11) due to the lack of evidence to support them at the time of these versions release. Also, AS is progressively vanishing as an eponym.55 However, as mentioned in the introduction, recent genomic data shows a differential genetic architecture in AS in comparison with other DSM-IV subtypes of ASD, with the former having a larger polygenic contribution. Therefore, we maintained DSM-IV terminology for the purpose of the present work.
We also encountered a significant correlation between paternal and maternal age at conception in line with previous findings56; and a modest significant correlation between paternal and maternal autistic traits, in line with assortative mating mechanisms.57,58 These correlations make it difficult to disentangle possible sex-specific effects of parental age at conception. However, previous works have observed that advanced maternal age increases the risk for autism in the offspring regardless of paternal age, but not vice versa,59 which further supports the hypothesis that paternal de novo variation and maternal polygenic contribution increase the risk of ASD in the offspring.
Both mothers and fathers reported lower scores than British population reference values in the self-rated AQ questionnaire.42 In light of our results, it is possible that the higher proportion of AS cases in the British sample could contribute to the higher AQ scores in their parents. Even so, in the absence of Spanish reference values it is difficult to interpret this result. We need to wait until Spanish reference values are available to understand the differences between the two populations. We must also take into account that the AQ in the undiagnosed population, in our case parents of individuals with ASD, is possibly not measuring the same ASD traits as it would do in the ASD population, but rather an extended autism phenotype.60
One of the main limitations of this study is the self-reported nature of autistic traits assessment and the absence of a measure of cognitive abilities in parents, which probably influences the way they see themselves and also modulates the risk for conceiving high or low-functioning ASD subjects. The absence of actual data on psychiatric comorbidities in the parents is also a limitation. Finally, the main limitation is the small sample size, which precludes, among other problems, applying multiple comparisons correction and the inclusion of offspring sex as a variable to be considered. Therefore, the analyses should be rendered exploratory and the results preliminary. Further studies using different and larger cohorts must be performed in order to have a to confirm these results and overcome the main limitation of the available sample size.
ConclusionsThe results of this study suggest that there is a relationship between autistic traits and advanced age at conception in mothers. Parental age seems to correlate with an Asperger Syndrome profile within an ASD offspring sample, rather than an autism or PDD profile. PRS seems to be only modestly associated with autistic traits in mothers of subjects with AS. All in all, a higher polygenic maternal contribution (both by AQ and PRS analyses) is a risk factor for an Asperger phenotype in the offspring.
Authors’ contributionsA.G.A., J.G.P. and M.P. designed research, with some contribution from the rest of authors; A.G.A. and J.G.P. performed research and data analysis with some contribution from rest of authors; A.G.A. and J.G.P. wrote the paper. All authors reviewed the manuscript.
Conflict of interestThe authors do not report any conflict of interest related to this work.
This work was supported by the Spanish Ministry of Science, Innovation and Universities, Instituto de Salud Carlos III (FIS PI14/02103 and FIS PI17/00819), co-financed by ERDF Funds from the European Commission, “A way of making Europe”, CIBERSAM., Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), European Union Structural Funds and European Union Seventh Framework Program and H2020 Program; Fundación Familia Alonso, Fundación Alicia Koplowitz and Fundación Mutua Madrileña. From the Hospital Universitario Gregorio Marañón we are grateful to patients and families who kindly participated in this project. Exome sequencing was performed at the Mount Sinai Research Center, New York, United States of America, as our ASD cohort is part of the Autism Sequencing Consortium (ASC) sample. AGA has been a recipient of a pre-doctoral fellowship (Formación de Profesorado Universitario FPU) from Spanish Ministry of Education, Culture and Sport (ref.: FPU16/01740), and this article is part of her PhD studies.