Neutrophil/lymphocyte (NLR), monocyte/lymphocyte (MLR), and platelet/lymphocyte (PLR) ratios, and systemic inflammatory index (SII) represent peripheral markers of inflammation associated with different severe mental disorders.
Material and methodsIn this study, these parameters were analyzed in a sample of 622 participants [197 patients with major depressive disorder (MDD), 154 with bipolar disorder (BD), 176 with schizophrenia (SCH), and 95 healthy controls (HC)]. Sociodemographic and clinical data of patients were recorded.
ResultsDifferences in age and sex were detected among groups (p<0.001), with SCH patients being younger and MDD patients being older. After stratifying by sex, these ratios were compared using the nonparametric ANCOVA (Quade's test) using age as a covariate. In males, no significant statistical differences were found between groups. However, differences were observed in MLR in the subgroup of females [MDD: 0.23 (SD=0.09); BD: 0.23 (SD=0.11); SCH: 0.24 (SD=0.11); HC: 0.29 (SD=0.13); F=5.376, p=0.001]. Post hoc testing revealed that there are MLR differences between HC versus MDD and between HC versus BD, with higher values in HC versus the other two groups. On the other hand, no differences were found in either males or females for any of the studied ratios, among the three diagnostic groups.
ConclusionsMLR is reduced in MDD and BD patients versus HC, but exclusively in the female group. However, based on the analyzed indices, it is not possible to differentiate among the three diagnostic groups of patients. As a limitation of this study, note that the effects of psychopharmacological treatments and smoking have not been controlled for.
The hypothesis that inflammatory processes are involved in psychiatric disorders has repeatedly been reported in the scientific literature.1,2 There is growing evidence supporting the role of immune and inflammatory pathways in the pathophysiology of psychotic and mood disorders, as seen in the identification of systemic inflammatory biomarkers in the peripheral blood of patients suffering from these disorders.3 Nevertheless, the relationship between mental health and low-grade inflammation is also influenced by lifestyle factors such as substance use, diet, gut dysbiosis, physical activity, and chronic stress, as well as physical comorbidities.1,4,5
In addition to biomarkers that require specialized or expensive techniques for their detection, such as cytokines, others have been identified that are easily accessible, inexpensive, and potentially clinically relevant, based on complete blood counts or routine clinical analytics.6 For example, systemic inflammatory biomarkers such as neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR), systemic immune-inflammatory (SII) index, red cell distribution width (RDW), C-reactive protein/albumin ratio (CAR), and neutrophil/albumin ratio (NAR) have been proposed in different studies.7,8 The ratios calculated from the white blood cell count parameters obtained from complete blood counts have proven to be useful indicators of inflammation, because it is the balance of the proportions of each component that may indicate, better than one single parameter, a state of inflammation and its severity. With this rationale, NLR, MLR, and PLR ratios have been widely used as biomarkers of inflammation in various pathological conditions and can provide prognostic information in various diseases, including cardiovascular disease, infections, and cancer.9,10 Despite being nonspecific, these ratios can guide treatment decisions and monitor effectiveness of treatment over time, predicting disease progression and risk of complications.
NLR, MLR, and PLR have been evaluated in schizophrenia (SCH), schizoaffective disorders, and mood disorders, with elevated ratios compared with healthy controls (HC).7,11,12 Recently, elevated NLR has been found in patients with a first episode of psychosis (FEP) compared with HC and could represent a prognostic marker as it has been shown to have a lower value in FEP patients who reached remission in a 2-year follow-up study.13 In addition, it has been suggested that these ratios might be useful tools to identify patients with BD who are at risk for metabolic syndrome.14
Haematopoietic imbalance, as extrapolated from complete blood counts, has been reported in patients with a major depressive episode, and this imbalance appears more severe when suicidal behavior co-occurs.15 NLR has been postulated recently as a biomarker of suicidal behavior in a cohort of depressive patients.16 On the other hand, PLR has been correlated with severity of depression with inconsistent results for other indexes.17
Furthermore, the SII index incorporates three crucial parameters of the immune response and may be more accurate than the previously mentioned paired ratios, as it includes neutrophils (primary immune response, they increase in inflammatory processes), lymphocytes (specialized effector cells, they increase in targeted secondary immune responses), and platelets (very important players at the crossroads of hemostasis and the immune response).18 Although less studied, SII has been postulated as a risk factor for depression in patients with diabetes mellitus.18
Although these biomarkers (SII, NLR, MLR, and PLR) have been thoroughly studied in various psychiatric disorders, to our knowledge, there are no comparative studies that analyze all four of them in bipolar disorder (BD), schizophrenia (SCH), and major depressive disorder (MDD).
The aim of this study is to analyze differences in the aforementioned biomarkers in patients with MDD, BD, and SCH versus HC and to identify potential differences in these markers among the three groups of patients.
Material and methodsStudy sampleWe performed a cross-sectional study, including 622 Caucasian participants≥18 years recruited in the Mental Health Area of Oviedo, Spain. All participants gave informed consent. The study was conducted according to the World Medical Association Declaration of Helsinki (2013).
Participants were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5): MDD (n=197), BD (n=154), and SCH (n=176). Our control population consisted of 95 healthy active blood donors to the regional blood bank.
Exclusion criteria were acute infection, active or chronic inflammatory or autoimmune diseases, current treatment with anti-inflammatory or immunosuppressant drugs, acute coronary syndrome, history of chronic renal, hepatic, or cerebrovascular disease, and hematological disorders.
Clinical assessmentPsychometric evaluation included the Spanish versions of different scales and questionnaires. We employed the Hamilton Depression Rating Scale (HDRS) to determine severity of depression (patients with diagnosis of MDD/BD); the Young Mania Rating Scale (YMRS) to evaluate the presence and severity of mania symptoms (BD); the Positive and Negative Syndrome Scale (PANSS) to determine severity of psychotic symptoms, and the Calgary Depression Scale for Schizophrenia (CDSS) to assess the presence of depressive symptoms (SCH).
Suicide attempt (SA) is defined by the American Psychiatric Association (2013) as a “self-initiated sequence of behaviors by an individual who, at the time of initiation, expected that the set of actions would lead to his or her own death”.
Complete blood count analysesFasting blood samples were collected from the cephalic vein in EDTA tubes between 8:00 and 9:00am. Complete blood counts (CBC) were performed the same day using a Sysmex XN-10/XN-20 haematology analyser, which is an FDA-cleared automated haematology analyser, capable of obtaining complete blood counts (including white blood cell differential) from blood samples by fluorescent flow cytometry using a semi-conductor laser and hydrodynamic focusing in dedicated channels. The neutrophil, lymphocyte, monocyte, and platelet counts (which are given in 103/μL, as a unit) were used to calculate the neutrophil/lymphocyte (NLR), monocyte/lymphocyte (MLR), and platelet/lymphocyte (PLR) ratios. Additionally, the systemic immune-inflammation index (SII) was calculated as (platelet×neutrophil)/lymphocyte count.
Statistical analysisData were analyzed using IBM SPSS Statistics for Windows, v24.0. (Armonk, NY: IBM Corp.). A Kolmogorov–Smirnov normality test was used to determine if variables were normally distributed. A Chi-square (χ2) test was used to compare categorical variables and frequencies. A one-way analysis of variance (ANOVA) with Tukey's post hoc test was used to compare normally distributed variables between groups. However, a Kruskal–Wallis test was performed to analyze any abnormally distributed variables. Bivariate correlations were performed to determine associations between ratios and sociodemographic and clinical characteristics. Based on previous data, sex-stratified analyses were performed.10 Then, inflammatory ratios were compared using a nonparametric analysis of covariance (ANCOVA) (Quade's test) to adjust for covariates (age), with Tukey's post hoc test. Statistical significance was set at α=0.05 (two-sided).
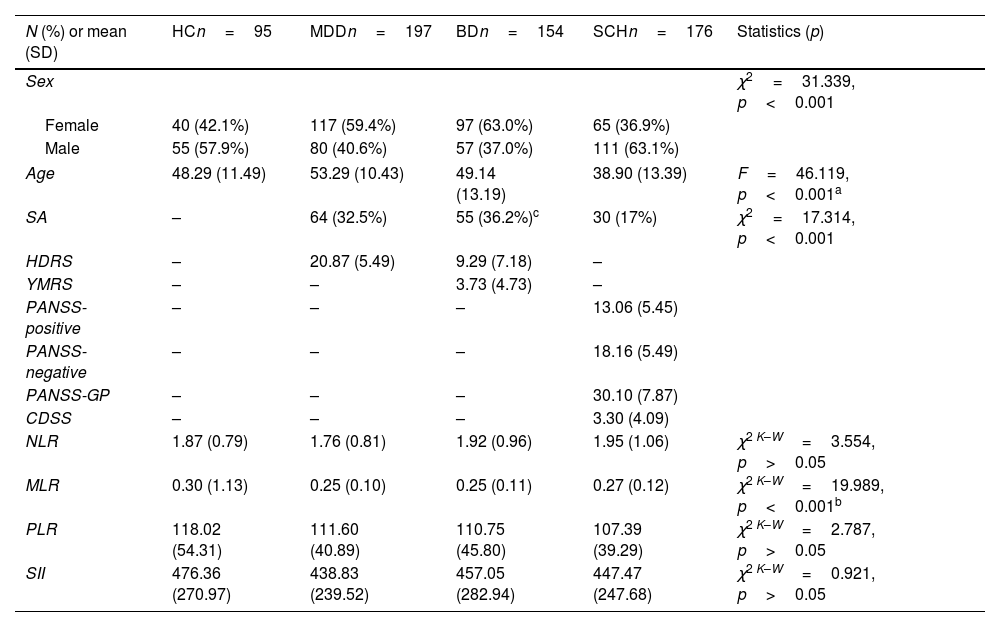
ResultsDescription of the sampleThe final sample included 622 participants of whom 95 were HC. The clinical and sociodemographic characteristics are shown in Table 1.
Sociodemographic and clinical data of the sample.
| N (%) or mean (SD) | HCn=95 | MDDn=197 | BDn=154 | SCHn=176 | Statistics (p) |
|---|---|---|---|---|---|
| Sex | χ2=31.339, p<0.001 | ||||
| Female | 40 (42.1%) | 117 (59.4%) | 97 (63.0%) | 65 (36.9%) | |
| Male | 55 (57.9%) | 80 (40.6%) | 57 (37.0%) | 111 (63.1%) | |
| Age | 48.29 (11.49) | 53.29 (10.43) | 49.14 (13.19) | 38.90 (13.39) | F=46.119, p<0.001a |
| SA | – | 64 (32.5%) | 55 (36.2%)c | 30 (17%) | χ2=17.314, p<0.001 |
| HDRS | – | 20.87 (5.49) | 9.29 (7.18) | – | |
| YMRS | – | – | 3.73 (4.73) | – | |
| PANSS-positive | – | – | – | 13.06 (5.45) | |
| PANSS-negative | – | – | – | 18.16 (5.49) | |
| PANSS-GP | – | – | – | 30.10 (7.87) | |
| CDSS | – | – | – | 3.30 (4.09) | |
| NLR | 1.87 (0.79) | 1.76 (0.81) | 1.92 (0.96) | 1.95 (1.06) | χ2 K–W=3.554, p>0.05 |
| MLR | 0.30 (1.13) | 0.25 (0.10) | 0.25 (0.11) | 0.27 (0.12) | χ2 K–W=19.989, p<0.001b |
| PLR | 118.02 (54.31) | 111.60 (40.89) | 110.75 (45.80) | 107.39 (39.29) | χ2 K–W=2.787, p>0.05 |
| SII | 476.36 (270.97) | 438.83 (239.52) | 457.05 (282.94) | 447.47 (247.68) | χ2 K–W=0.921, p>0.05 |
SD, standard deviation; HC, healthy controls; MDD, major depressive disorder; BD, bipolar disorder; SCH, schizophrenia; SA, suicide attempts; HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; -GP, -General Psychopathology; CDSS, Calgary Depressive Symptom Scale; NLR, neutrophil/lymphocyte; MLR, monocyte/lymphocyte; PLR, platelet/lymphocyte ratios; SII, systemic inflammatory index.
Differences in age (F=46.119, p<0.001) and sex (χ2=31.339, p<0.001) were detected among groups, with SCH patients being younger and MDD patients being older. Also, there was a higher proportion of males in the SCH group, while females were predominant in the MDD and BD groups (Table 1).
Regarding lifetime history of SA, significant differences were observed among the three diagnostic groups (χ2=17.314, p<0.001), with BD patients presenting a greater percentage of SA (36.2%).
Regarding symptoms, the MDD group presented a moderate to severe score on the HDRS, which was considerably lower in the BD group since this group included 64 (41.6%) euthymic patients (YMRS≤6 and HDRS≤7). All MDD patients had HDRS scores≥8 at assessment and in most cases (88.8%)≥15. In the SCH group, patients were clinically stable, presenting slightly higher negative symptom scores and lower positive and depressive symptom scores. Complete, comprehensive clinical data are shown in Table 1.
Inflammatory ratios and their relationship with sociodemographic factors and clinical severityThe ratio values of each group are presented in Table 1. Significant differences were observed among the four groups only for MLR (χ2K–W=19.989, p<0.001). Post hoc analysis revealed significant differences between MDD versus HC (p=0.001), BD versus HC (p<0.001), and SCH versus HC (p=0.033).
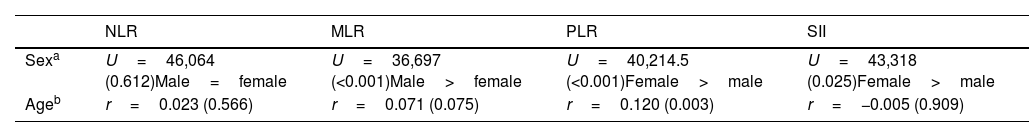
Taking the sample as a whole, age significantly correlated only with PLR (r=0.120, p=0.003). Females presented higher levels of PLR than males [116.17 (42.81) versus 105.92 (44.72), t=−2.921, p=0.004], but lower levels of MLR [0.24 (0.10) versus 0.29 (0.12), t=4.795, p<0.001]. Complete data are shown in Table 2.
In the MDD group, the HDRS score negatively correlated only with PLR (r=−0.155, p=0.03). In the SCH and BD groups, the indices did not correlate with the clinical scores.
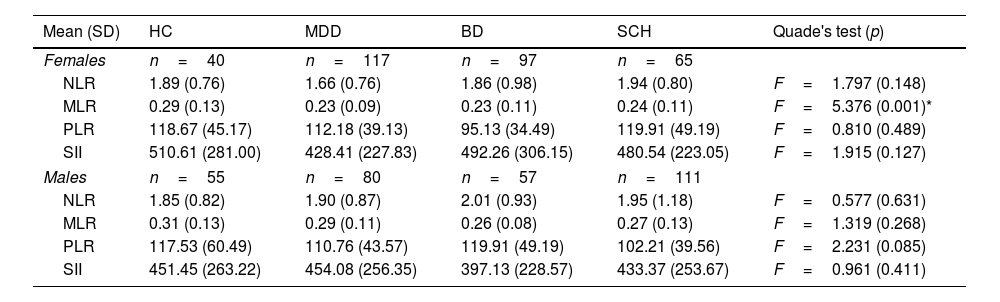
Inflammatory ratios in each diagnostic group stratified by sexAfter stratifying by sex, NLR, MLR, PLR, and SII ratios were compared using a Quade's test using age as a covariate. In males, no differences were found between MDD, BD, and SCH patients and HC. However, statistically significant differences were observed in MLR in the subgroup of females [MDD: 0.23 (SD=0.09); BD: 0.23 (SD=0.11); SCH: 0.24 (SD=0.11); HC: 0.29 (SD=0.13); F=5.376, p=0.001]. Post hoc testing showed MLR differences between HC versus MDD and between HC versus BD, with higher values in HC versus the other two groups. No differences were found in males or females for any of the studied ratios, among the three diagnostic groups (Table 3).
Inflammatory ratios in each diagnostic group stratified by sex.
| Mean (SD) | HC | MDD | BD | SCH | Quade's test (p) |
|---|---|---|---|---|---|
| Females | n=40 | n=117 | n=97 | n=65 | |
| NLR | 1.89 (0.76) | 1.66 (0.76) | 1.86 (0.98) | 1.94 (0.80) | F=1.797 (0.148) |
| MLR | 0.29 (0.13) | 0.23 (0.09) | 0.23 (0.11) | 0.24 (0.11) | F=5.376 (0.001)* |
| PLR | 118.67 (45.17) | 112.18 (39.13) | 95.13 (34.49) | 119.91 (49.19) | F=0.810 (0.489) |
| SII | 510.61 (281.00) | 428.41 (227.83) | 492.26 (306.15) | 480.54 (223.05) | F=1.915 (0.127) |
| Males | n=55 | n=80 | n=57 | n=111 | |
| NLR | 1.85 (0.82) | 1.90 (0.87) | 2.01 (0.93) | 1.95 (1.18) | F=0.577 (0.631) |
| MLR | 0.31 (0.13) | 0.29 (0.11) | 0.26 (0.08) | 0.27 (0.13) | F=1.319 (0.268) |
| PLR | 117.53 (60.49) | 110.76 (43.57) | 119.91 (49.19) | 102.21 (39.56) | F=2.231 (0.085) |
| SII | 451.45 (263.22) | 454.08 (256.35) | 397.13 (228.57) | 433.37 (253.67) | F=0.961 (0.411) |
SD, standard deviation; HC, healthy controls; MDD, major depressive disorder; BD, bipolar disorder; SCH, schizophrenia; NLR, neutrophil/lymphocyte; MLR, monocyte/lymphocyte; PLR, platelet/lymphocyte ratios; SII, systemic inflammatory index.
We aimed to identify specific or differential systemic inflammatory biomarkers of the main psychiatric diagnoses. However, except for the MLR biomarker, no differences in NLR, PLR, and SII were found between the different diagnostic groups compared with healthy individuals. In the case of MLR, a significantly lower value was observed in all patient groups versus HC. After taking age and sex into account, only females with BD and MDD showed differences in this index when compared with HC. On the other hand, this biomarker does not differentiate among the three diagnostic groups. However, other factors related to lifestyle, substance use, psychopharmacological treatments, and BMI have not been controlled for in this study.
Despite the fact that these indices reflect an inflammatory state and the vast majority of studies have found differences between patients and the healthy population,11,19 the results are not always consistent.
In patients with MDD, a recent meta-analysis concluded that inflammatory ratios, especially NLR, were significantly associated with an increased risk of depression, with a potential effect of sex-related differences on NLR values.20 No significant results were obtained for MLR values in this meta-analysis, as only three studies had analyzed this parameter, finding contradictory outcomes.20 Recently, a study in depressive adolescents found that monocyte count and MLR tended to be higher in comparison with HC but not significantly so, and significantly higher in those with suicide attempts.21 However, our results are not always in alignment with reported findings, and a previous study with our sample of depressive patients was the only one to find a reduction in MLR in patients versus HC.15 In this sense, the reduced peripheral MLR detected in MDD (and BD) patients could be related to recruitment of activated monocytes to the central nervous system, becoming active players in the neuro-inflammation described in these disorders.22 SII has been less studied but postulated as a risk factor for depression in patients with diabetes mellitus.18
Regarding patients with BD, previous studies reported higher NLR, MLR, and PLR in manic phases and subsequently lower after acute treatment remission, although they remained somewhat higher than controls.23,24 These findings suggest that they may be useful as state markers of relapses, but not so useful in the stable phases, and they are consistent with the idea that inflammation, in general terms, is more pronounced in decompensation states. Instead, they may be useful as a predictor of phase change in BD. For example, when only euthymic bipolar subjects are considered, no significance differences have been observed in either NLR or PLR.11 In this sense, analyses by subgroups suggest an influence of the bipolar phase. However, we did not find elevated inflammatory ratios in our BD sample with 41.6% of euthymic patients, nor after a stratified analysis with euthymic and non-euthymic groups (see Supplementary Material).
Our study also performed a stratified analysis by sex, which found that this lower MLR value in patients with diagnoses of MDD or BD was a finding specific to the female group. The normal ranges of the different cellular blood components are different depending on sex, and so are the shifts caused by an inflammatory status. The dynamics of these shifts are also sex-dependent in affective disorders, as we have previously observed in a smaller cohort of MDD patients where females with MDD presented significant lower monocyte counts.15 As this study suggests, MDD females were prone to develop a cumulative increase in hematological changes associated with systemic inflammation.15 Nevertheless, there is still inconclusive data about the potential effect of sex hormones on the immune system in patients with affective disorders, as concluded by the Lombardo et al. systematic review.25 Recently, Fusar-Poli et al.26 studied NLR, MLR, and PLR comparing different phases of bipolar disorder, concluding that PLR may represent the strongest biomarker of (hypo)mania regardless of sex, while NLR was significantly increased only in (hypo)manic males.
In our sample, contrary to other authors,19,27 we further found no differences in the group of patients with SCH after adjusting for age and sex, possibly because these patients were in outpatient follow-up receiving antipsychotic treatment. Other factors such as substance use, sedentary lifestyle, and poor dietary habits, which could have a negative influence on the pro-inflammatory state, have not been adequately controlled for; however, they would be expected to be more prevalent in patients.28,29 A recent longitudinal study of FEP found lower mean NLR values in patients who reached clinical remission versus the non-remitted group, with no effect of tobacco use or psychopharmacological treatments on the NLR.13
Lastly, our study did not include patients with anxiety disorders as a diagnostic category to compare with the other groups. To the best of our knowledge, there are no comparative studies in this regard, but recent studies in children and adolescents with anxiety disorders also identify an elevation of these markers (NLR, MLR, PLR) in this group of patients compared with the healthy population.30 Also, high NLR and PLR values may be associated with suicidal behavior in young patients with anxiety disorders or major depression.31 Furthermore, SII was significantly associated with depression or anxiety symptoms in patients with tuberculosis.32
At the transdiagnostic level, we did not find any differences among the three diagnostic groups. It is worth mentioning that the recent study by Bulut et al.,33 analysing differences in NLR and PLR in different psychiatric diagnoses identified a decrease in inflammation from the BD manic phase to SCH, bipolar disorder depression, and MDD. MLR is an index that has been analyzed in very few studies.19,24,34 Özdin et al.24 identified higher MLR in SCH and BD mania phase patients compared with HC, and higher NLR and MLR values in SCH compared with BD.
It should be noted that cross-comparison of the different studies is complicated, as patients may be in different stages of their disease and treatment. This suggests that conflicting findings might not be entirely rooted in the pathophysiology of the disease. However, more rigorous characterization and, potentially, longitudinal studies, are needed to dive deeper into the clinical usefulness of these “new generation” nonspecific inflammation biomarkers.
The SII parameter, which reflects immune response and systemic inflammation based on peripheral lymphocyte, neutrophil, and platelet counts, is a very infrequently studied marker. Although it has been used in other disorders, it has rarely been studied in psychiatric disorders.18,35 Recently, Dionisie et al.35 found that SII was higher (as was NLR) in bipolar depression than in unipolar depression. However, our results do not support the clinical utility of this parameter. It is thus necessary to explore the value of this index (SII) in larger cohorts, as it could provide additional information, due to its additional complexity compared with paired ratios.
Some limitations should be mentioned. Firstly, this is a cross-sectional study, which does not allow conclusions to be drawn about the impact of these markers from a longitudinal perspective. Furthermore, we did not consider the effects of antidepressants and other psychopharmacological treatments. Some studies have observed an anti-inflammatory effect with specific antidepressant and antipsychotic treatments and a consequent reduction in inflammatory indexes.36,37 However, some studies reported by other authors did not find any difference in the ratios analyzed between patients taking antipsychotics and those who were not.13,19 In addition, a recent study found that polytherapy, whether antidepressants or mood stabilizers, did not affect the NLR value.13 Smoking may be considered another confounding factor due to its effect on NLR elevation, and it was not assessed in our study.19 Although a large number of physical comorbidities were exclusion criteria in our study, other lifestyle-related factors (physical activity, diet, other substance use) that may have an influence on the inflammatory state were also not adequately controlled for. Another limitation is that we included mostly stable SCH outpatients, and a high proportion of BD patients were in an euthymic phase. It has been observed that the indices tend to approach normal ranges during stable phases.38 Although the association of clinical severity with these parameters has been analyzed in each diagnostic group, unfortunately, there is no transdiagnostic measure that assesses clinical severity among the different diagnoses. Nevertheless, the main strength of this article is that it compares the three main diagnoses of BD, SCH, and MDD, and to our knowledge, it is the first study to include MLR and SII in making this comparison.
ConclusionsIn conclusion, our data suggest that the MLR is reduced in MDD and BD patients compared with HC, but exclusively in the female group. However, the analyzed indices do not differentiate among the three diagnostic groups of patients. Although there is evidence of the presence of low-grade inflammation phases in the three diagnoses, the widespread use of NLR, PLR, and MLR is still subject to serious concerns and inconsistencies. It therefore seems necessary to study these indexes in greater depth and to study and establish the differences between the phases of each disorder, duration of illness, and treatments.
Role of funding sourceThis research was partially supported by grants PI14/02029 and PI17/01433 (PI: PAS), PI13/02263 and PI16/01761 (PI: JB), PI11/02493 and PI14/02037 (PI: PGP) of the Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Fondos Europeos de Desarrollo Regional (FEDER), by the Government of the Principality of Asturias PCTI-2021-2023 IDI/2021/111, and by Fundación para la Investigación e Innovación Biosanitaria del Principado de Asturias (FINBA). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestGP has received honoraria for lecturing from Otsuka, Lundbeck and Janssen-Cilag.
LGB has received honoraria for lecturing and/or research or travel grants for attending conferences from the Spanish Foundation of Psychiatry and Mental Health, Instituto de Salud Carlos III, Otsuka, Lundbeck, Janssen-Cilag, Servier, Angelini and Pfizer.
PAS has been a consultant to and/or has received honoraria or grants from Adamed, CIBERSAM, European Commission, GlaxoSmithKline, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Plan Nacional Sobre Drogas and Servier.
PGP has been a consultant to and/or has received honoraria/grants from Angelini, Alianza Otsuka-Lundbeck, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Otsuka, and Pfizer.
JB has received research grants and served as consultant, advisor or speaker within de last 5 years for: AB-Biotics, Acadia Pharmaceuticals, Angelini, Casen Recordati, D&A Pharma, Exeltis, Gilead, GSK, Ferrer, Indivior, Janssen-Cilag, Lundbeck, Mundipharma, Otsuka, Pfizer, Reckitt-Benckiser, Roche, Sage Therapeutics, Servier, Shire, Schwabe Farma Ibérica, research funding from the Spanish Ministry of Economy and Competiveness – Centro de Investigación Biomedica en Red area de Salud Mental (CIBERSAM) and Instituto de Salud Carlos III-, Spanish Ministry of Health, Social Services and Equality – Plan Nacional sobre Drogas- and the 7th Framework Program of the European Union.
All other authors declare that they have no conflicts of interest.
The authors wish to thank Sharon Grevet for her English assistance.