Respiratory syncytial virus is the leading cause of hospital admission for bronchiolitis in Spain. Immunoprophylaxis with palivizumab is an effective measure in those patients who meet the criteria for its administration. The aim of our study was to evaluate whether the extension of these criteria would reduce the rate of hospitalisation for this reason.
MethodsA retrospective analytical observational case–control study was conducted in a tertiary hospital, including 338 neonates born between 2017 and 2022. The “controls” arm included patients with criteria for palivizumab administration according to the Spanish Society of Neonatology indications and the “cases” arm included preterm patients up to 35 weeks of gestational age excluded from these criteria. The main variables assessed were the occurrence of RSV-positive bronchiolitis and the need for admission in both groups.
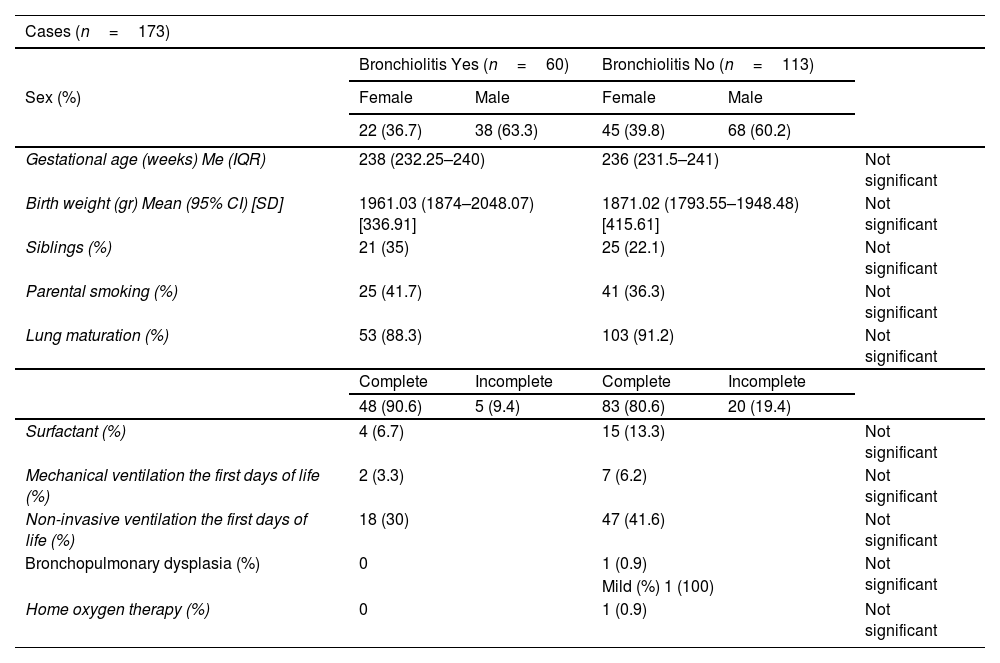
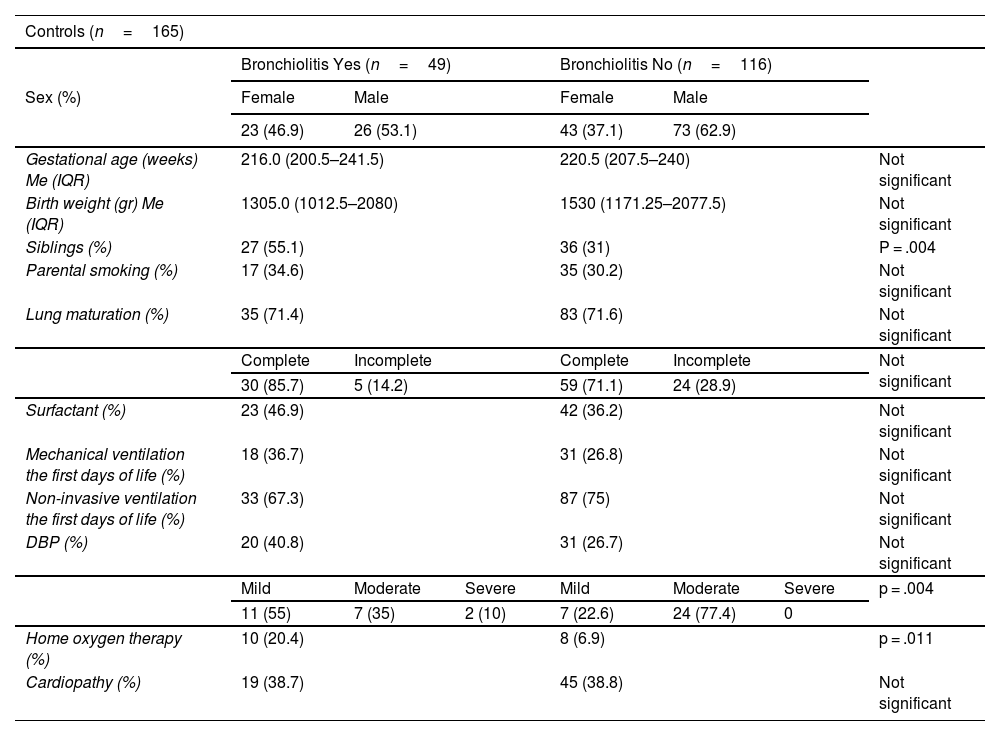
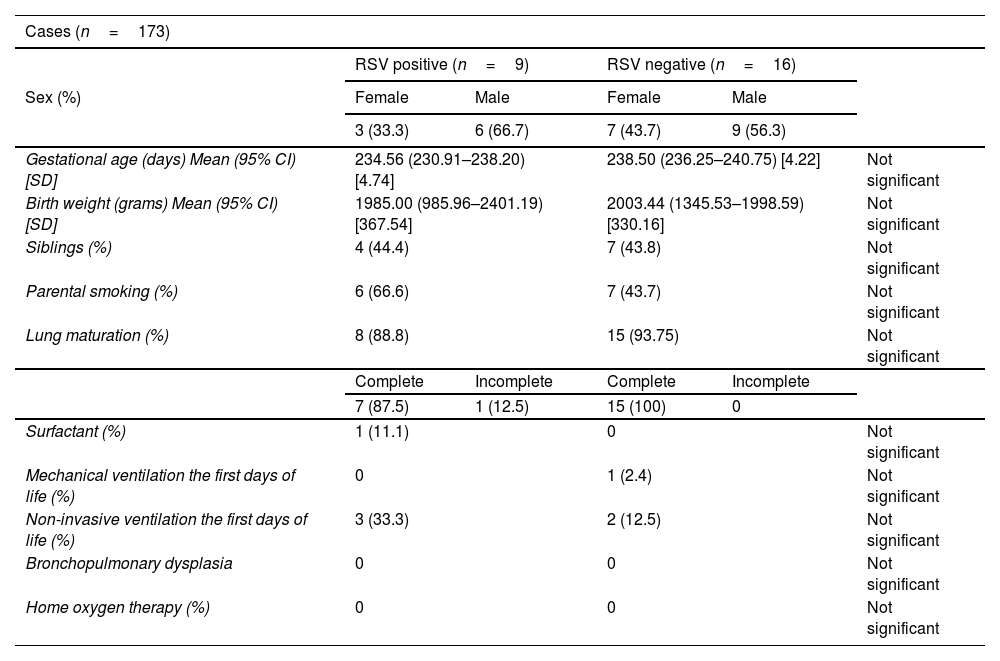
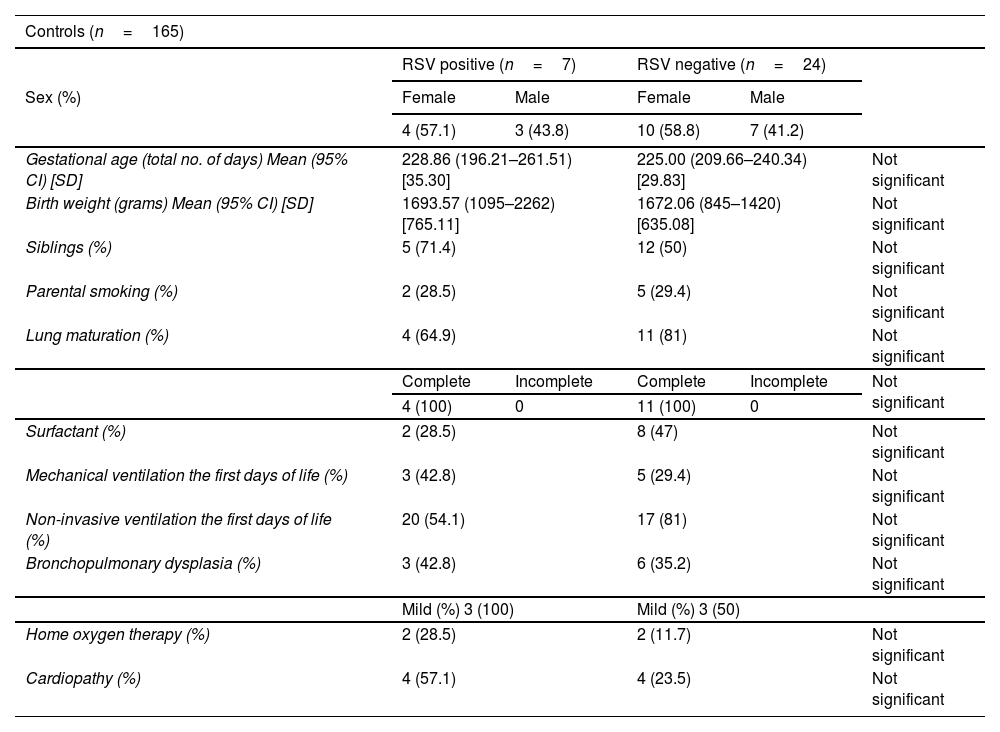
ResultsThe incidence of RSV bronchiolitis and admission to the hospital ward in the case group was similar to that found in our palivizumab-immunised population (control group).
Statistically significant differences were only found for the presence of school-aged siblings as a risk factor for the development of bronchiolitis.
ConclusionsAn extension of the criteria for palivizumab administration to all preterm infants younger than 35 weeks would lead to increased costs without a reduction in infection rates and respiratory syncytial virus admissions.
El virus sincitial respiratorio supone la primera causa de ingreso hospitalario por bronquiolitis en nuestro medio. La inmunoprofilaxis con palivizumab es una medida efectiva en aquellos pacientes que cumplen criterios para su administración. El objetivo de nuestro estudio es evaluar si la ampliación de dichos criterios disminuiría la tasa de hospitalización por este motivo.
MétodosSe realizó un estudio observacional analítico retrospectivo de tipo casos y controles desarrollado en un hospital de tercer nivel, que incluía 338 neonatos nacidos entre los años 2017 a 2022. En el brazo «controles» se incluyeron pacientes con criterios de administración de palivizumab según indicaciones de la Sociedad Española de Neonatología y en el brazo «casos», pacientes prematuros de hasta 35 semanas de edad gestacional excluidos de estos criterios. Se evaluaron como variables principales la aparición de bronquiolitis VRS positiva y la necesidad de ingreso en ambos grupos.
ResultadosLa incidencia de bronquiolitis por virus respiratorio sincitial y el ingreso en planta de hospitalización en el grupo de casos fue similar a la encontrada en nuestra población inmunizada con palivizumab (grupo control).
Solo se encontró diferencia estadísticamente significativa en cuanto a la presencia de hermanos en edad escolar como factor de riesgo para el desarrollo de bronquiolitis.
ConclusionesUna ampliación de criterios de administración de palivizumab a todos los prematuros menores de 35 semanas conllevaría un aumento de costes sin implicar una reducción de las tasas de infección ni de ingresos por virus respiratorio sincitial.
Artículo
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora