To explore the implications for use of the different vaccine packaging options, in terms of sustainability, safety, and nurses' preferences; as well as to elaborate a proposal for main concepts of vaccine packaging that could serve as a user guide for nurses.
MethodsA literature review has been carried out about logistic, ergonomic, and practical aspects of the different vaccine packaging options. The search was performed in PubMed, CINAHL, and MEDLINE databases. Initially, 99 sources were identified, of which 19 articles were included for review.
Review/ResultsThe findings have been grouped in 4 categories: description of vaccine packaging options, and implications in sustainability, safety, and nurses' preferences in clinical practice.
Conclusions and implications for practiceNationwide, there are just a few studies addressing logistic, ergonomic, and practical aspects of the different vaccine packaging options. Regarding the review process of packaging formats, a descriptive section within the document has been developed by experts, including a summary chart. There exists knowledge about liquid formulations, in particular, the prefilled-syringe and multi-dose formats. In relation to sustainability, in terms of packaging, cold storage, and transport, among others; we consider that these are main aspects that should be addressed by its contribution to the reduction of environmental impact; given its scarce presence in the current literature.
Explorar las implicaciones de los formatos de presentación de las vacunas en su uso, en términos de sostenibilidad, la seguridad y la percepción de las enfermeras; así como elaborar una propuesta de los conceptos fundamentales de presentación de vacunas que puedan servir de guía de uso para enfermeras.
Material y métodosSe llevó a cabo una revisión de la literatura en busca de información sobre aspectos logísticos, ergonómicos y prácticos de los distintos formatos de presentación de las vacunas. La búsqueda se diferentes bases de datos. Se incluyeron 19 artículos en la revisión.
Desarrollo/ResultadosSe han agrupado los hallazgos en cuatro categorías: formatos de presentación de las vacunas, sostenibilidad, seguridad y preferencia por las enfermeras en la práctica clínica.
Conclusiones e implicaciones para la prácticaA nivel nacional, existen escasos estudios que versen de los aspectos logísticos, ergonómicos y prácticos de los distintos formatos de presentación de las vacunas. En cuanto a la revisión de los distintos formatos de presentación, se ha elaborado un apartado específico en el documento por profesionales expertas en la materia y una figura resumen. Existe conocimiento acerca de la formulación totalmente líquida, en concreto, con el formato de presentación de jeringa precargada y de las presentaciones multidosis y monodosis. Con respecto a la sostenibilidad, en términos de embalaje, almacenamiento en neveras y medio de transporte, entre otros; consideramos que es un aspecto fundamental que debiera ser tenido en cuenta por su contribución a la reducción en el impacto medioambiental; y que se encuentra escasamente desarrollado en la bibliografía existente.
Within the framework of systematic immunisation schedules, and the administration of recommended immunogens, regardless of their funding, the main objectives of immunisation are to enjoy good health throughout all life stages and not to relinquish autonomy as a person.1
Vaccination is one of the most effective and safest public health interventions. Immunisation or vaccination is one of the most commonly performed health processes throughout the life of a healthy person. It comes into play before a person's birth, with the vaccinating of a woman during pregnancy, then later with the infant, continuing during childhood and adolescence, reaching adulthood and continuing throughout old age. Indeed, it is present at all stages of life.2 It is also used for sick people with risk factors due to underlying diseases, chronic diseases, or in special situations of vulnerability. Immunisation also represents a safe and effective benefit in the prevention of complications.3,4
In Spain, nurses play an essential role in the promotion and implementation of immunisation, for which they require adequate training and information for its development.5
Vaccination timing is one of the key situations for the appearance of errors. Among the most frequent errors are those related to the administration of the wrong vaccine and those associated with an incorrect indication of the vaccine.6 Nurses must therefore be familiar with the presentation characteristics of vaccines for proper handling, administration, and disposal. As a result, the existence of protocols or strategies aimed at avoiding errors in the handling and administration of immunogens is essential. In keeping with this approach, this study differentiates 2 priority objectives: to explore the implications of the presentation formats of vaccines in use, in terms of sustainability, safety, and the preferences of use reported by nurses and to develop a proposal regarding the essential concepts of the types of vaccine presentations that can serve as a guide for use by nurses.
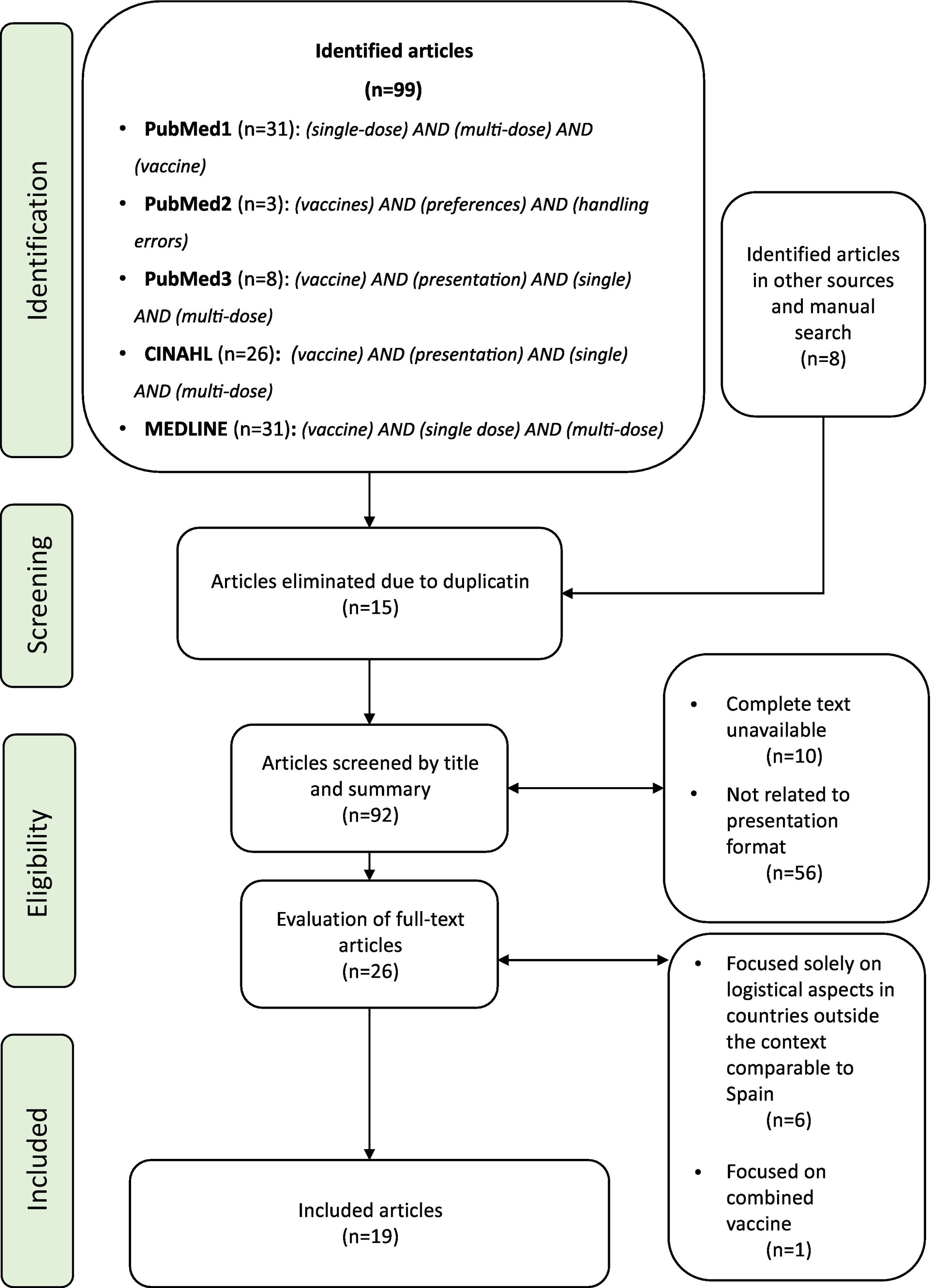
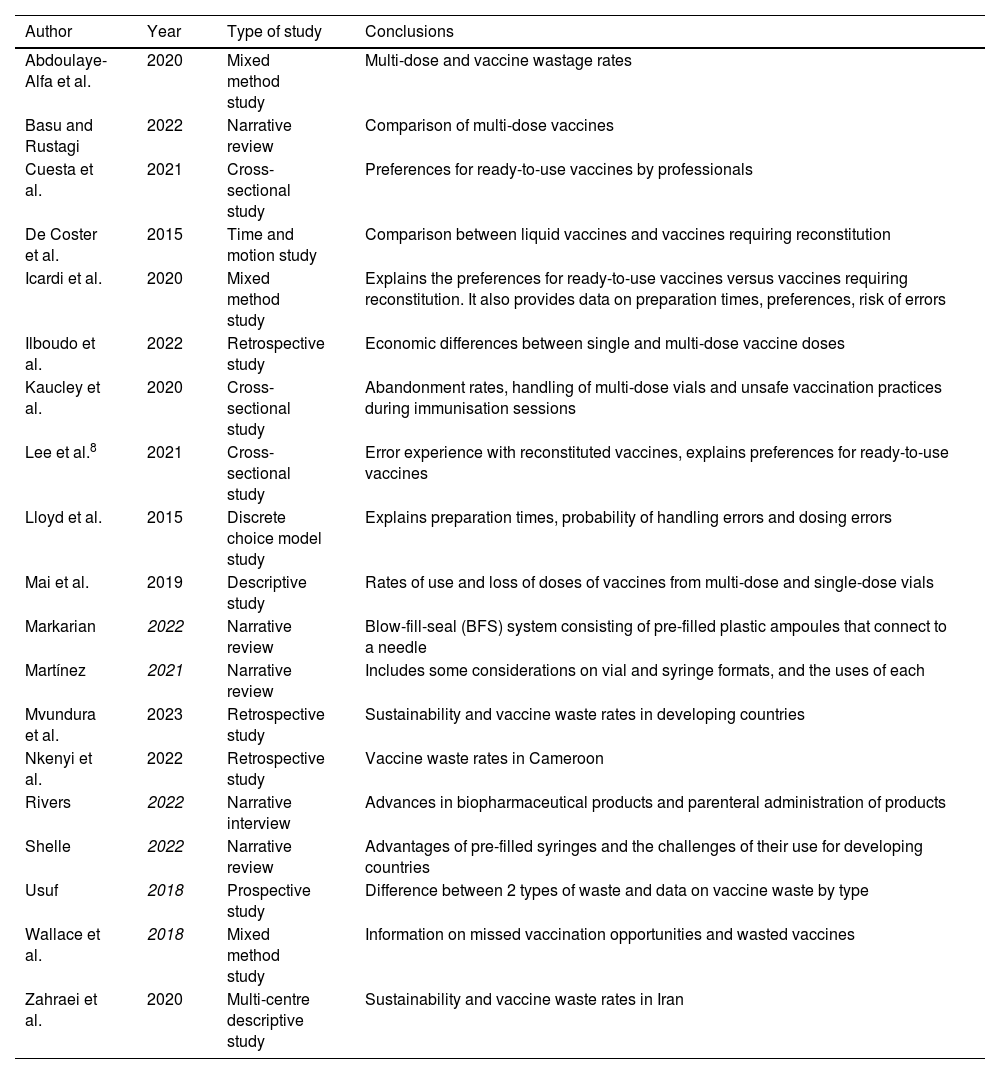
Material and methodsA narrative review was conducted related to the logistical, ergonomic, and practical aspects of the different presentation formats of vaccines. To this end, a search was carried out in PubMed, CINAHL, and MEDLINE for the relevant literature published in English and Spanish from 2018 to 2023. Of the former, those available in full text, related to the presentation formats, were chosen. In a subsequent phase, those that appeared focused solely on logistical aspects in countries outside the context comparable to Spain and those studies that were focused on combined vaccines were excluded. The search terms were contained in the title/abstract and focused on the vaccine presentation. The search strategy and the process of including articles are contained in Fig. 1. The design of the included studies is highly varied and provides different levels of evidence: randomised clinical trial (1), observational studies (8), survey-based studies (6), and review articles (4). Most of the primary sources (14) are based on studies carried out in European countries (8), with only one carried out in Spain. The articles obtained and their brief description are represented in Table 1. The literature review led to grouping the findings into 4 main aspects: vaccine presentation formats; their sustainability; their safety, and nurses' preferences in clinical practice.
Search algorithm performed and inclusion of articles, based on the protocol used for publication of panoramic review, PRISMA-ScR.7
Search results.
| Author | Year | Type of study | Conclusions |
|---|---|---|---|
| Abdoulaye-Alfa et al. | 2020 | Mixed method study | Multi-dose and vaccine wastage rates |
| Basu and Rustagi | 2022 | Narrative review | Comparison of multi-dose vaccines |
| Cuesta et al. | 2021 | Cross-sectional study | Preferences for ready-to-use vaccines by professionals |
| De Coster et al. | 2015 | Time and motion study | Comparison between liquid vaccines and vaccines requiring reconstitution |
| Icardi et al. | 2020 | Mixed method study | Explains the preferences for ready-to-use vaccines versus vaccines requiring reconstitution. It also provides data on preparation times, preferences, risk of errors |
| Ilboudo et al. | 2022 | Retrospective study | Economic differences between single and multi-dose vaccine doses |
| Kaucley et al. | 2020 | Cross-sectional study | Abandonment rates, handling of multi-dose vials and unsafe vaccination practices during immunisation sessions |
| Lee et al.8 | 2021 | Cross-sectional study | Error experience with reconstituted vaccines, explains preferences for ready-to-use vaccines |
| Lloyd et al. | 2015 | Discrete choice model study | Explains preparation times, probability of handling errors and dosing errors |
| Mai et al. | 2019 | Descriptive study | Rates of use and loss of doses of vaccines from multi-dose and single-dose vials |
| Markarian | 2022 | Narrative review | Blow-fill-seal (BFS) system consisting of pre-filled plastic ampoules that connect to a needle |
| Martínez | 2021 | Narrative review | Includes some considerations on vial and syringe formats, and the uses of each |
| Mvundura et al. | 2023 | Retrospective study | Sustainability and vaccine waste rates in developing countries |
| Nkenyi et al. | 2022 | Retrospective study | Vaccine waste rates in Cameroon |
| Rivers | 2022 | Narrative interview | Advances in biopharmaceutical products and parenteral administration of products |
| Shelle | 2022 | Narrative review | Advantages of pre-filled syringes and the challenges of their use for developing countries |
| Usuf | 2018 | Prospective study | Difference between 2 types of waste and data on vaccine waste by type |
| Wallace et al. | 2018 | Mixed method study | Information on missed vaccination opportunities and wasted vaccines |
| Zahraei et al. | 2020 | Multi-centre descriptive study | Sustainability and vaccine waste rates in Iran |
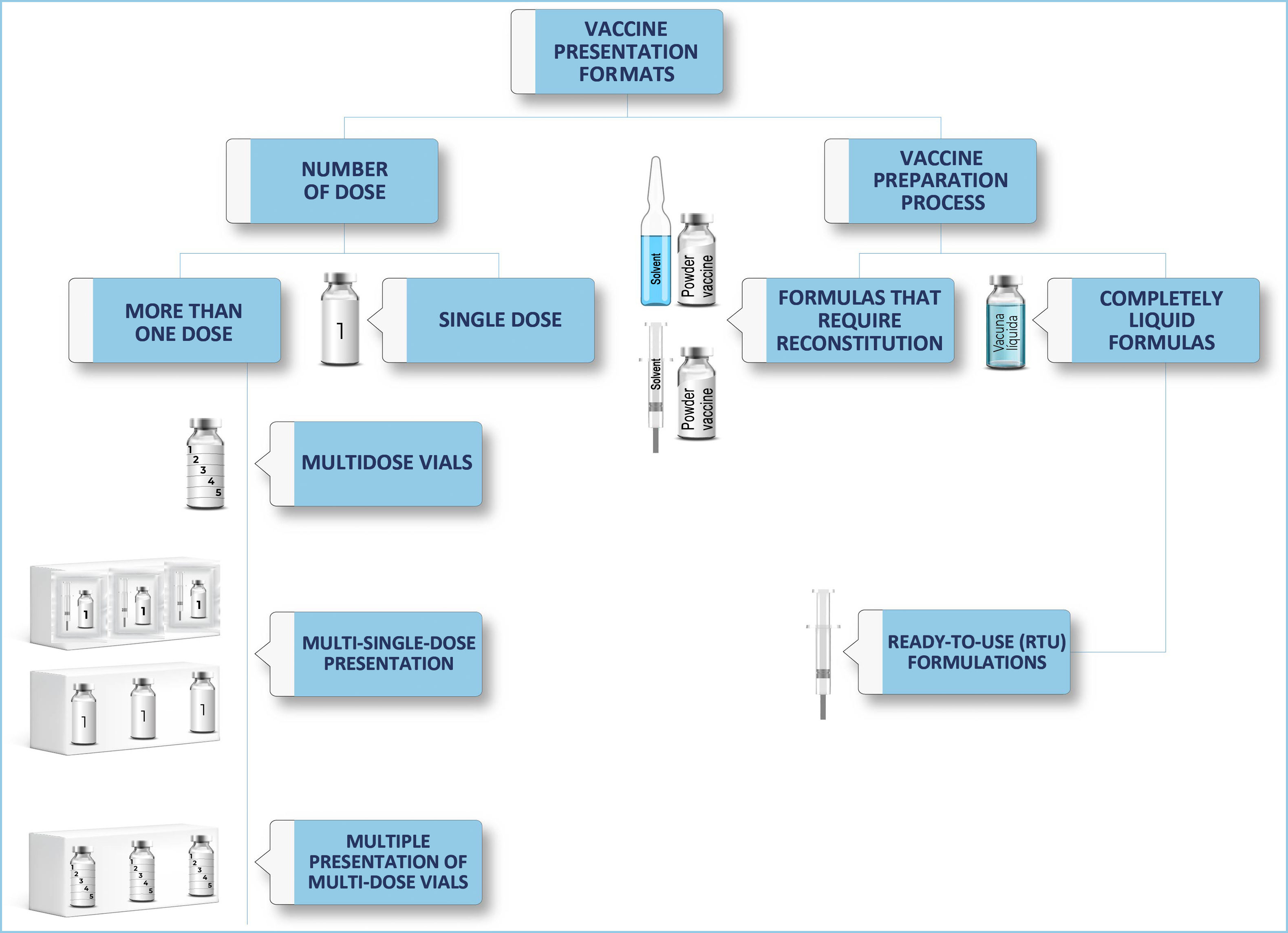
From the perspective of the pharmacotechnics of vaccines for parenteral use, they can be classified based on the number of doses provided by a single presentation, or on the preparation process of the drug before its administration. This classification is represented in Fig. 2, which proposes the fundamental concepts in the presentation of vaccines, which can serve as a guide for nurses in their care practice.
According to RD 175/20017 of February 23, which approves the rules for the correct preparation and quality control of magistral formulas and official preparations, the formulations are protected by packaging material, which may be primary or secondary depending on whether or not it is in contact with the product. The primary packaging is the packaging that is in contact with the product and protects it directly (the vial or the pre-filled syringe). The secondary packaging is intended to create a loading unit that helps in the reservation and transfer of the product (blister or external box).
According to the first classification based on the number of doses, we call a single-dose vaccine that presentation intended to administer a single dose to a patient.
If, on the other hand, with a single specialty we can administer more than one dose, we will find the following formats.
Multi-dose vials: We will call those vaccines in which the same vial contains several doses.
Multi-single-dose presentation: Those whose secondary packaging contains several individualised doses. There are presentations with a greater or lesser amount of secondary packaging on the market. Each of these doses is presented independently in its primary packaging (vials, pre-filled syringes).
And, a combination of the two above is the multi-dose presentation, whose secondary packaging contains several multi-dose vials.
Depending on the process of preparing the vaccine for administration, we can talk about:
Formulas that require reconstitution: These are preparations in which, to reach the final drug intended to be administered, they require prior preparation, generally adding a solvent to a solute in solid or liquid form.
Completely liquid formulas: They do not require reconstitution. When the primary packaging of these formulas is a pre-filled syringe, we are talking about ready-to-use formulas (RTU).9
Taking these definitions into account, we will find vaccines, approved by the Spanish Medicines Agency, that combine these formats. For example, there are specialties in multi-dose vials that also require reconstitution, an example of this is ComirnatyOmicron XBB.1.5, with National Code (CN) 7 632 344 and registration number (NR) 1 201 528 019, fully liquid multi-dose vials such as MenQuadfi (presentation of 10 vials) CN 763017, Jcovden CN 7300038, and NR 1201525001; English: single-dose presentations with 2 vials, one containing the antigen powder and the other containing a suspension for reconstitution, and a syringe to be filled with the reconstituted suspension, such as Shingrix CN 7293040 and NR 1181272001. We also have single-dose vaccines that require reconstitution, in which the solvent is in a pre-filled syringe that draws up the solute from a vial, such as Proquad CN 6542767 and NR 05323010 or Stamaril CN 6542972 and NR 65098; ready-to-use vaccines, such as Boostrix Polio (CN 6504338, NR 6504338) or Hexyon (CN 7314998 and NR 1130829002), the latter also has a presentation that serves as an example to illustrate a multi-single dose, Hexyon CN 606140 and NR 113829007, whose outer packaging contains 10 pre-filled syringes.
Advantages and disadvantages in vaccine presentation formatsVaccines are traditionally packaged and distributed in vials containing single or multi-dose doses. This approach requires the healthcare professional to prepare the vaccine for injection, draw up the precise dose from the vial with a syringe, administer it to the patient, and store the remaining doses until the vial is empty. Potentially, there are differences in local practices, cultural preferences for certain pharmaceutical formulations and also in terms of vaccine delivery logistics in other countries, which may affect the preference for liquid vaccines.10 In the study by Basu et al.,11 they determined the choice of single- or multi-dose vial vaccine packaging type as a crucial element of vaccine coverage, as it is associated with the degree of vaccine wastage, cost effectiveness, logistics, cold chain, and potential safety concerns. In addition, a general economic advantage of multi-dose vials is noted, especially in the context of developing countries.
Pre-filled or RTU syringes are a more recent alternative, which continues to gain popularity among vaccine manufacturers and healthcare professionals, due to several advantages they provide in their routine use. RTU syringes contain the exact dose, reduce drug wastage, and are suitable for routine vaccination, such as influenza.12,13 These presentations have been shown to improve the effectiveness of the vaccination service, both for the health professionals involved, mainly nurses, and for the organisation itself. Some of the advantages they provide are as follows:12,14 They save time for health professionals during vaccine preparation; reduce administration costs; speed up and simplify administration; reduce dosing errors and product waste; minimise the potential for microbial cross-contamination and transmission of pathogens, which can occur in doses from the same vial; minimise the storage space required and decrease the amount of waste generated. Reducing steps in vaccine preparation decreases the opportunities for immunisation errors and could affect safety.10
SustainabilityRegarding sustainability, a possible global aspect is the rate of vaccine wastage. It is one of the critical quality indicators of an immunisation programme and is defined as the proportion of doses from opened or unopened vaccine vials that are not used for vaccination. According to reports from the World Health Organisation, vaccine wastage worldwide exceeds 50%.15 In this study, developed in Iran, they state that the concern about increased waste when opening multi-dose vials could limit access to vaccination services and contribute to low coverage and/or late protection of children by national vaccination programmes.11
Decreasing errors in vaccination can eliminate the need to prepare and use a new vaccine, which would result in less waste and indirectly contribute to sustainability.8
Regarding the higher acquisition costs of RTU vaccines, it has been reported that the higher costs are offset by lower administrative costs and greater safety, compared to vaccines in single- and multi-dose vials.14
Few studies on safety for the community and the environment exist. Multi-dose vials reduce waste generation with its consequent benefits in terms of environmental impact,11 although many of the vaccines are not available in multi-dose format. We consider that having a formulation/presentation that requires less packaging allows its primary size to be smaller and saves space, both in storage in refrigerators and in the required means of transport, in addition to minimising the waste generated. Presentations that are contained in sustainable packaging such as cardboard or with recyclable materials and without materials such as plastic blisters, should be more highly appreciated. Furthermore, another aspect to consider is the number of needles per dose of vaccine supplied. Adopting measures to reduce the supply of extra needles in the presentation of vaccines can avoid the use of raw materials and the generation of plastic waste.16 In short, the less packaging the vaccine has, both primary and secondary, the greater its contribution to controlling the impact of the environmental footprint.
SafetyThe prevention of accidental occupational exposures in healthcare professionals has been a long-standing demand by the healthcare community. Percutaneous injuries caused by cannulated needles are those associated with a higher risk of acquiring infections by serum-transmitted microorganisms.17 At a national level, Order ESS/1451/201318 for the prevention of injuries caused by sharp and piercing instruments in the healthcare and hospital sector, states that all needles must have a biosecurity mechanism. Some safety measures for nurses are syringes with a luer-lock system, to avoid accidental disconnections and punctures, and vaccines with anatomical dead space, which prevent product loss. RTU pre-filled syringes, which do not require reconstitution, reduce needle handling, and the consequent risk of accidental punctures.
A vaccine that requires less handling will reduce administration risks.10 Taking this into account, a fully liquid single-dose vaccine will offer fewer opportunities for error by reducing the risk of incorrect dosing, as there is no need to mix or aspirate the contents of the vials and therefore incomplete aspiration or reconstitution failure can be avoided.10 It is also known that errors during vaccine preparation could be divided into 2 main categories: errors that could lead to health complications and errors that reduce the effectiveness of immunisation. The preparation of the fully liquid vaccine may lead to fewer immunisation errors compared to the non-fully liquid vaccine, as the latter requires many more preparation steps. In addition, during the preparation of the injectable vaccine, if improper manipulations occur, sterility may be compromised, which could contaminate the product. In a South Korean study of 450 nurses,8 several types of safety-related errors were identified in vaccine administration, including problems with reconstitution (incomplete aspiration of the reconstitution vial, spillage or leakage during reconstitution), handling (deformation of the needle when inserted into the vial stopper), and administration (using the same needle for reconstitution and injection and administration of unreconstituted solvent). In this study, approximately half of the participants who detected reconstitution errors discarded them and prepared a new vaccine, increasing patient safety.
Some of the risk reduction strategies could include: training of health professionals, placing labels on storage containers, redesigning labelling, and packaging of vaccines.8
Preferences of nurses in clinical practiceHealth professionals value positively any approach, method, or system that prevents errors in the administration of vaccines. The incorporation of safety measures and quality control during the vaccination process also boosts confidence, both in the population and in health professionals, which, in turn, promotes vaccination rates and ensures the success of the immunisation programme. It is important that nurses participate and have a say in the decision-making process, similarly to other professionals involved in vaccination.14
Several studies,10,19–21 have demonstrated that nurses have a positive perception regarding the inclusion of vaccines with RTU presentation and even preference for these presentations.9 Some factors could be: the time required for preparation;8 the ability to reduce possible erroneous handling and dosage errors; the generation of waste per vaccination, and the scheduling of the time assigned to each vaccination in the vaccination services.14
The characteristics of RTU vaccines offer advantages in the management of vaccination appointments, allowing them to be stored if the vaccination appointment is postponed.9
However, vaccines that require reconstitution must be used immediately after vaccination, otherwise they must be discarded.
Conclusions and implications for clinical practiceThere are few national level studies that address logistic, ergonomic, and practical aspects of the different vaccine presentation formats.
Regarding the type of professionals involved in the act of vaccination, it should be noted that, in the international context, this includes doctors as well as nurses, although there may be differences between Spain and other countries.8,14,19
In the section “Presentation formats of vaccines”, content is provided on the different presentation formats of vaccines and their summary classification (Fig. 2), as a guide for nurses and other professionals involved in the vaccination process. Of note is that knowledge exists regarding the fully liquid formulation, specifically, with the RTU presentation format or pre-filled siringe.10,12–14 There is also information at an international level, analysing the benefits and disadvantages of multi- and single-dose vaccines.11
With regard to sustainability, speaking in terms of packaging, storage in refrigerators, and means of transport, among others, we consider that it is a fundamental aspect that should be taken into account for its contribution to reducing environmental impact and that is poorly developed in the existing literature. It is also worth mentioning the reduction in vaccination errors and, therefore, vaccine waste, as a relevant aspect in relation to sustainability.
According to several studies,9,10,19,21 it is clear that nurses have a positive perception regarding the inclusion of vaccines in RTU presentation. In short, we can conclude that RTU vaccines provide safety for the act of immunisation, save time in handling, flexibility in the time of use and reduce errors, which implies an increase in safety.
FundingThe authors external to the Spanish Institute of Nursing Research declare that they have received funding from the General Council of Official Colleges of Nursing of Spain for the preparation of this manuscript.
Please cite this article as: Domínguez-Fernández S, Forcada-Segarra JA, Cuesta-Esteve I, Fernández-Fernández S, Cáceres Fernández-Bolaños R, Fontán-Vinagre G, Guerrero-Menéndez R. Revisión de los formatos de presentación de vacunas y sus principales características. Vacunas. 2024. https://doi.org/10.1016/j.vacune.2024.10.001.