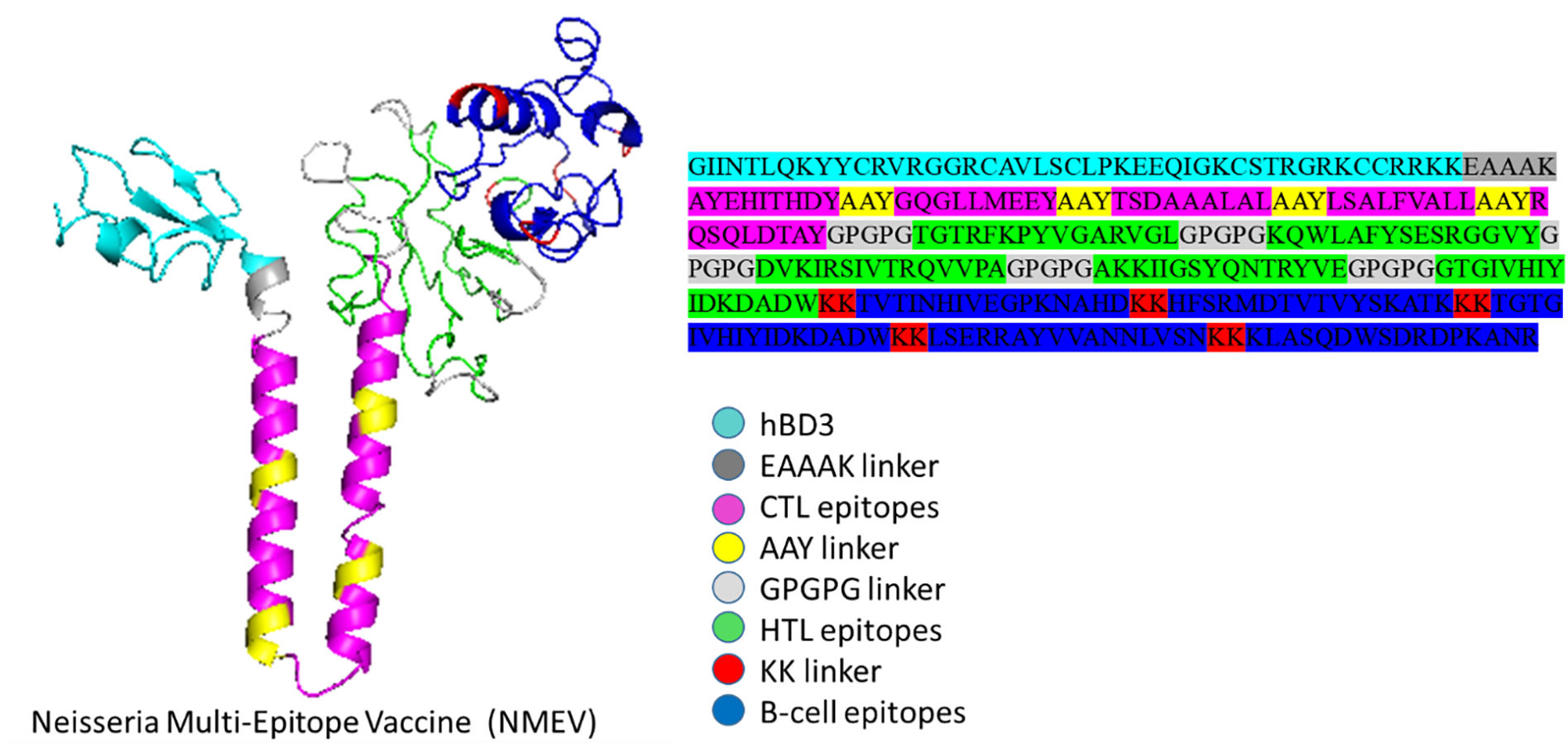
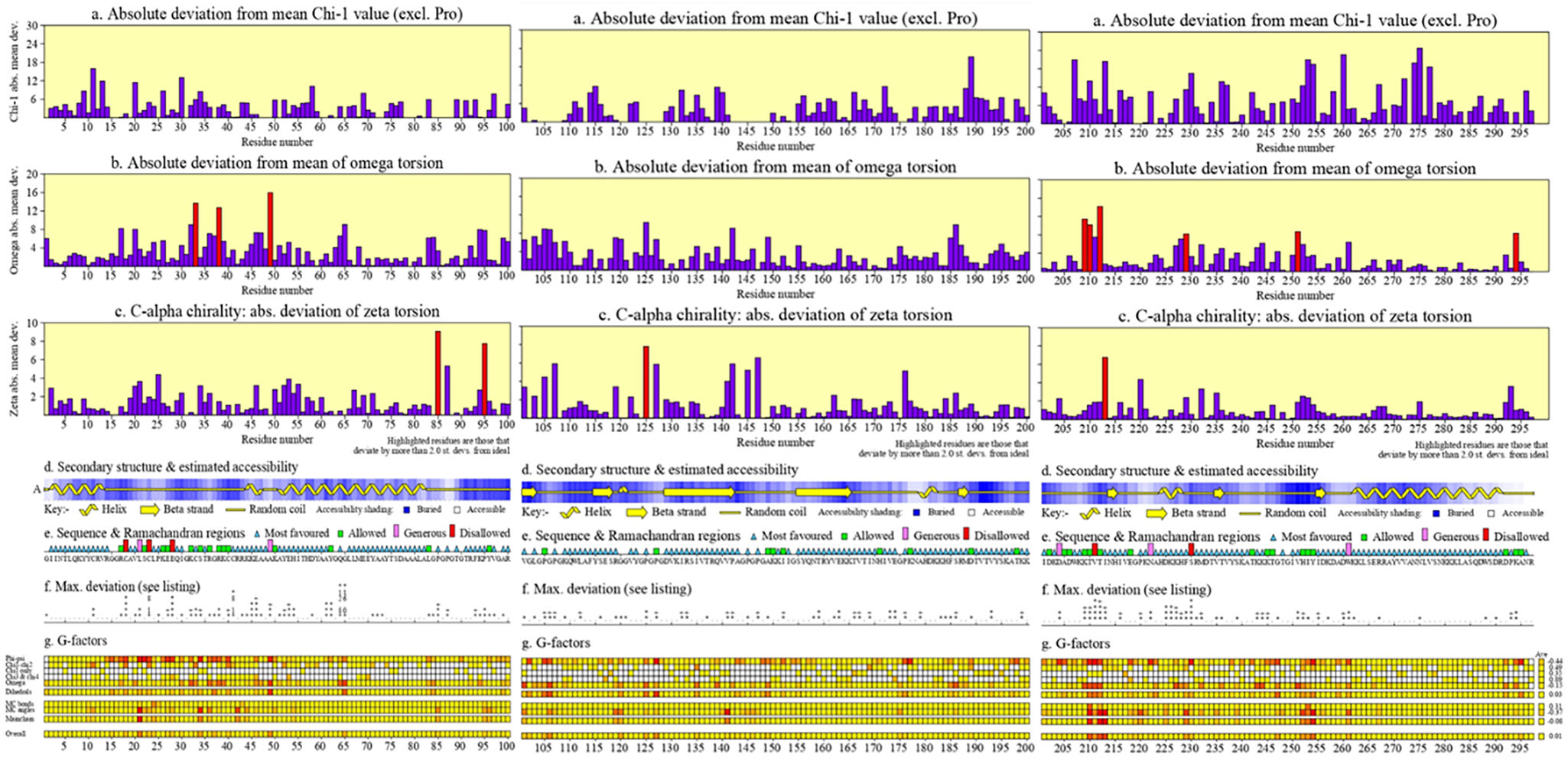
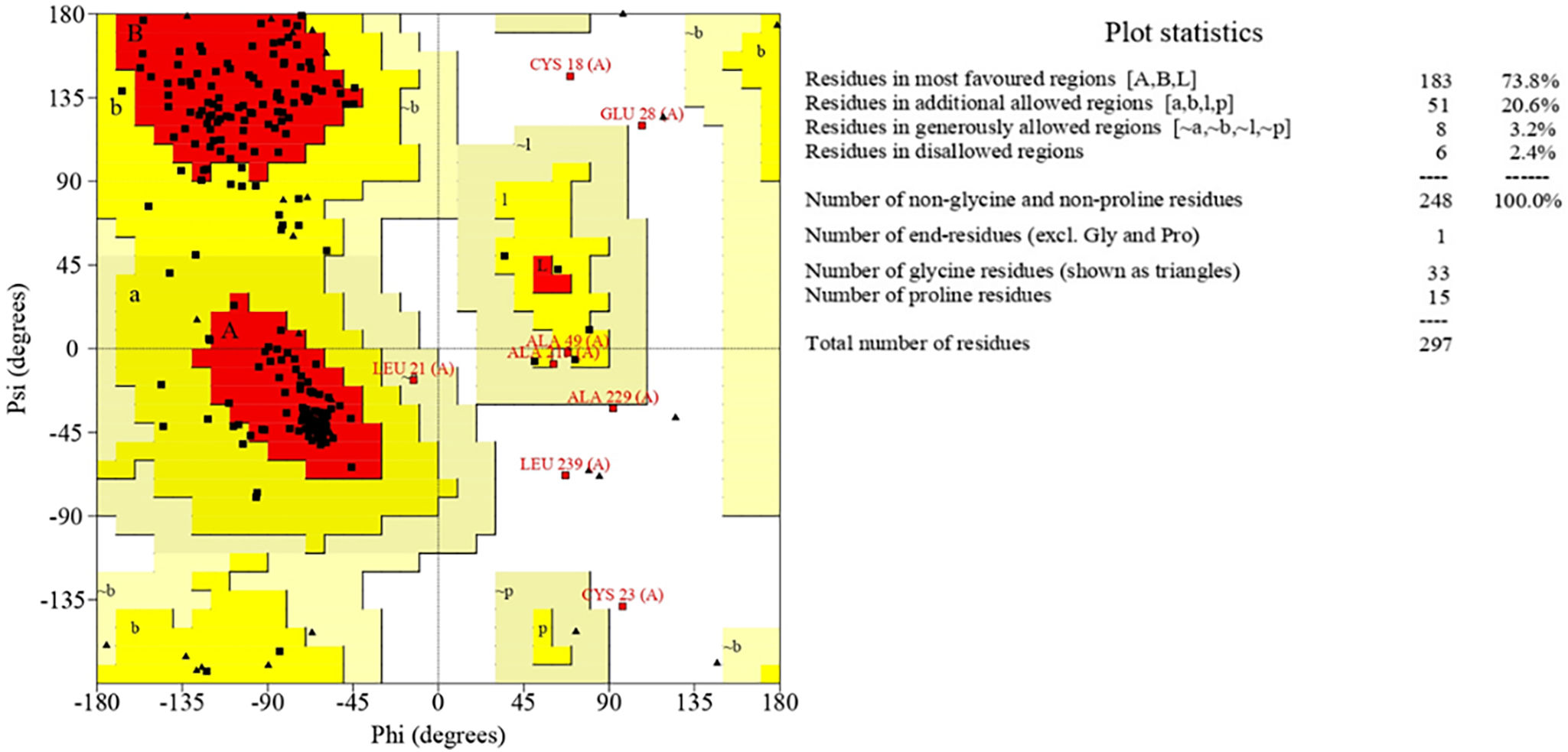
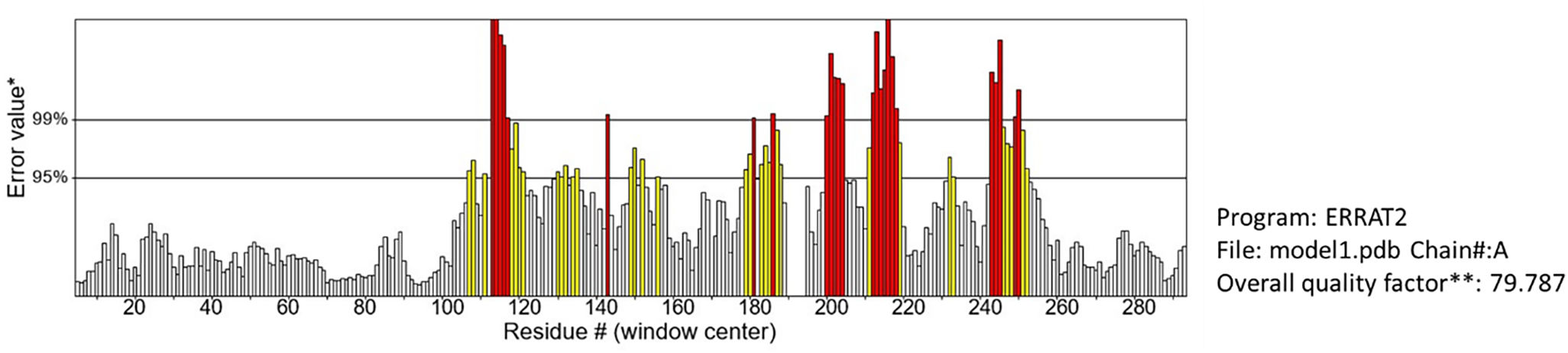
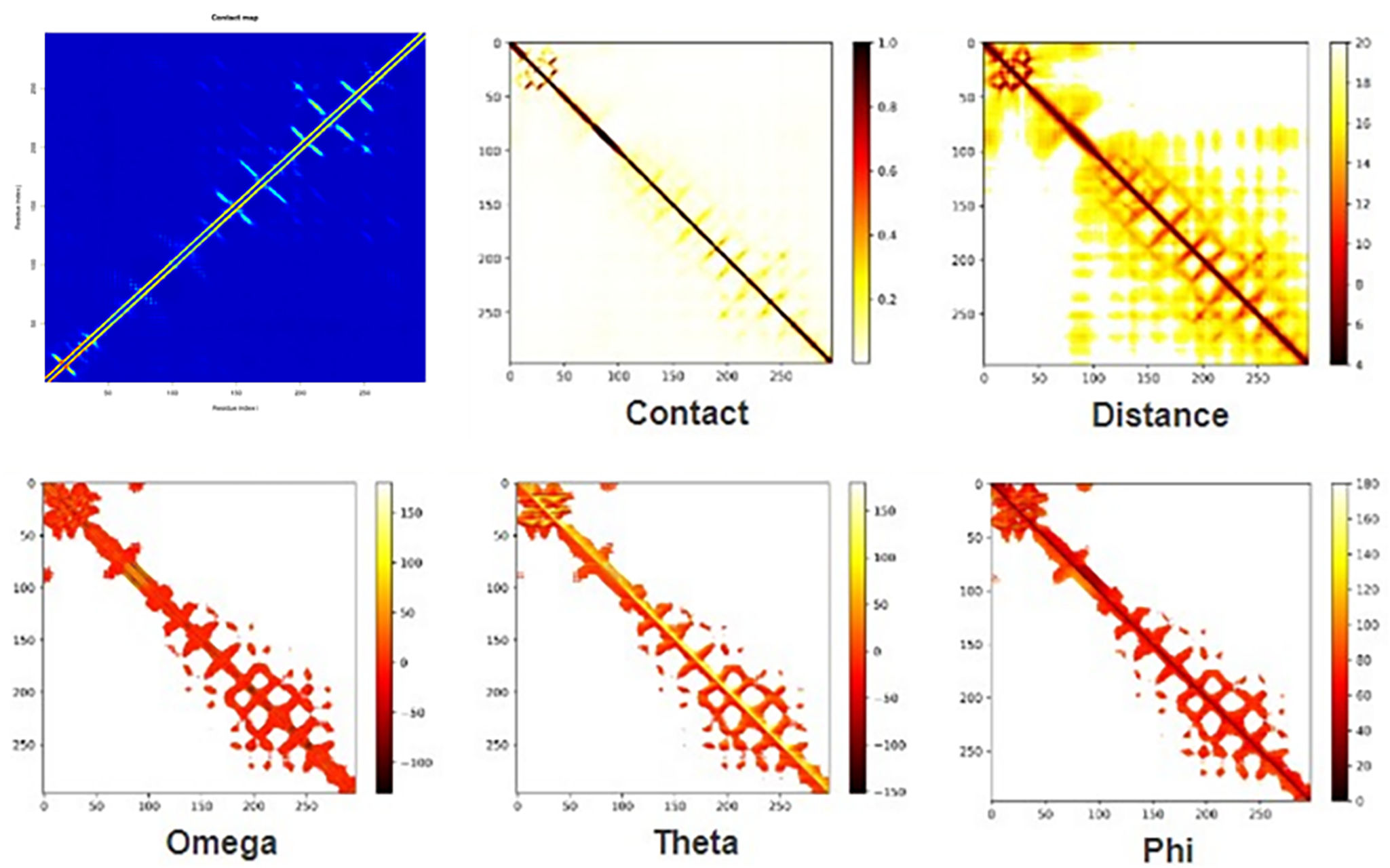
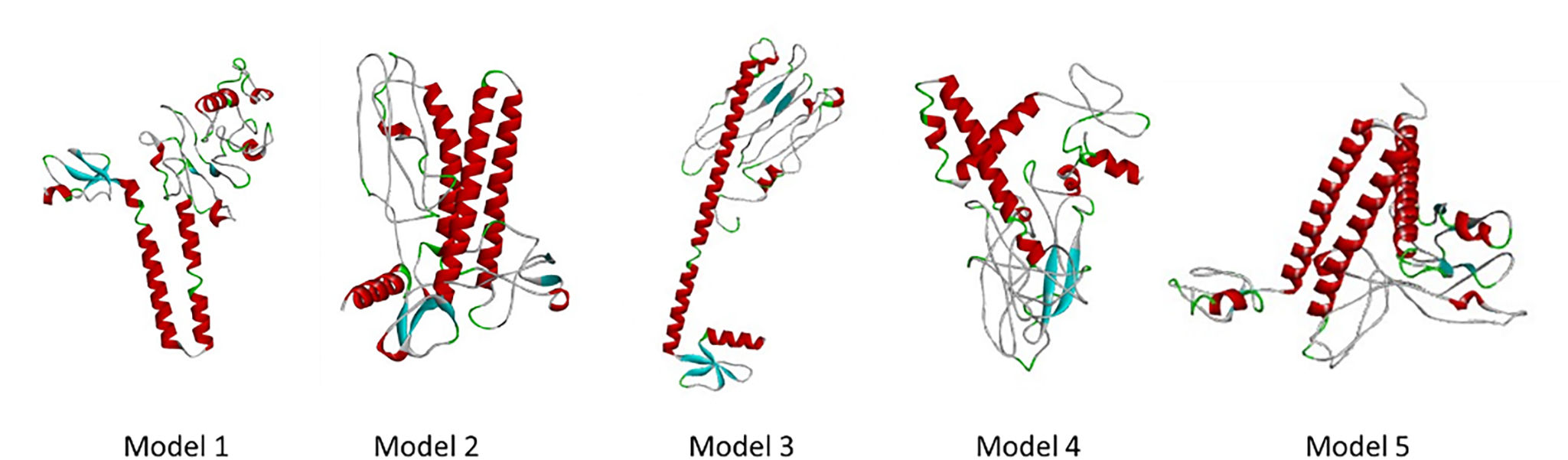
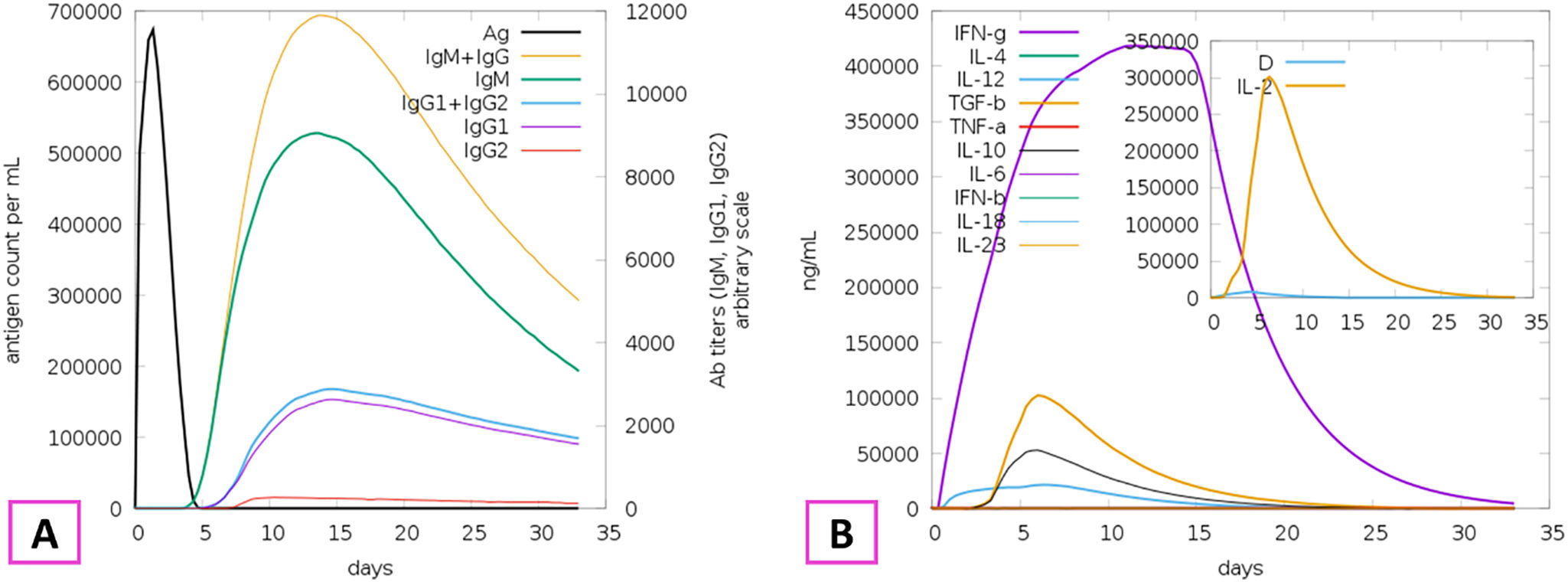
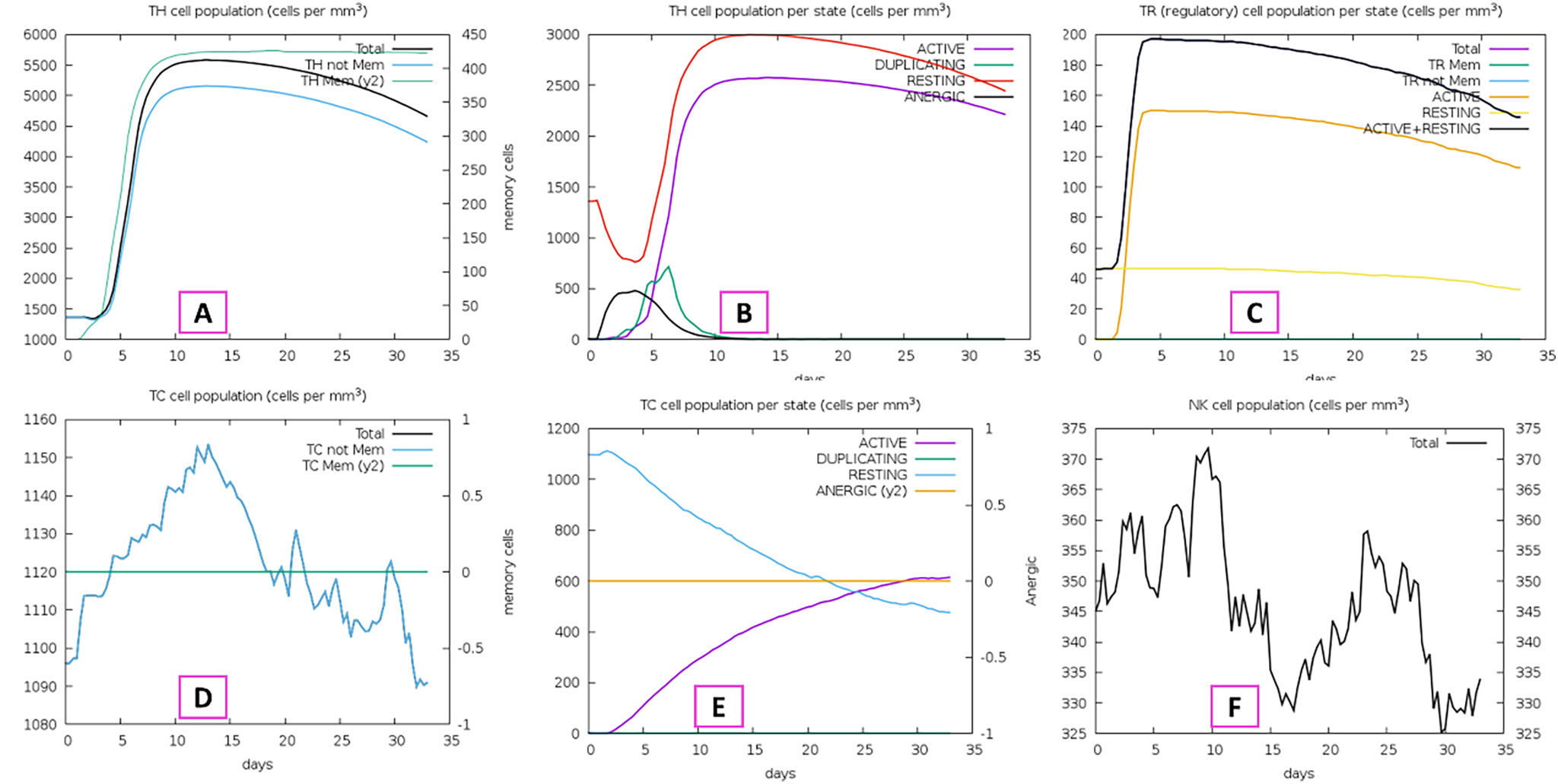
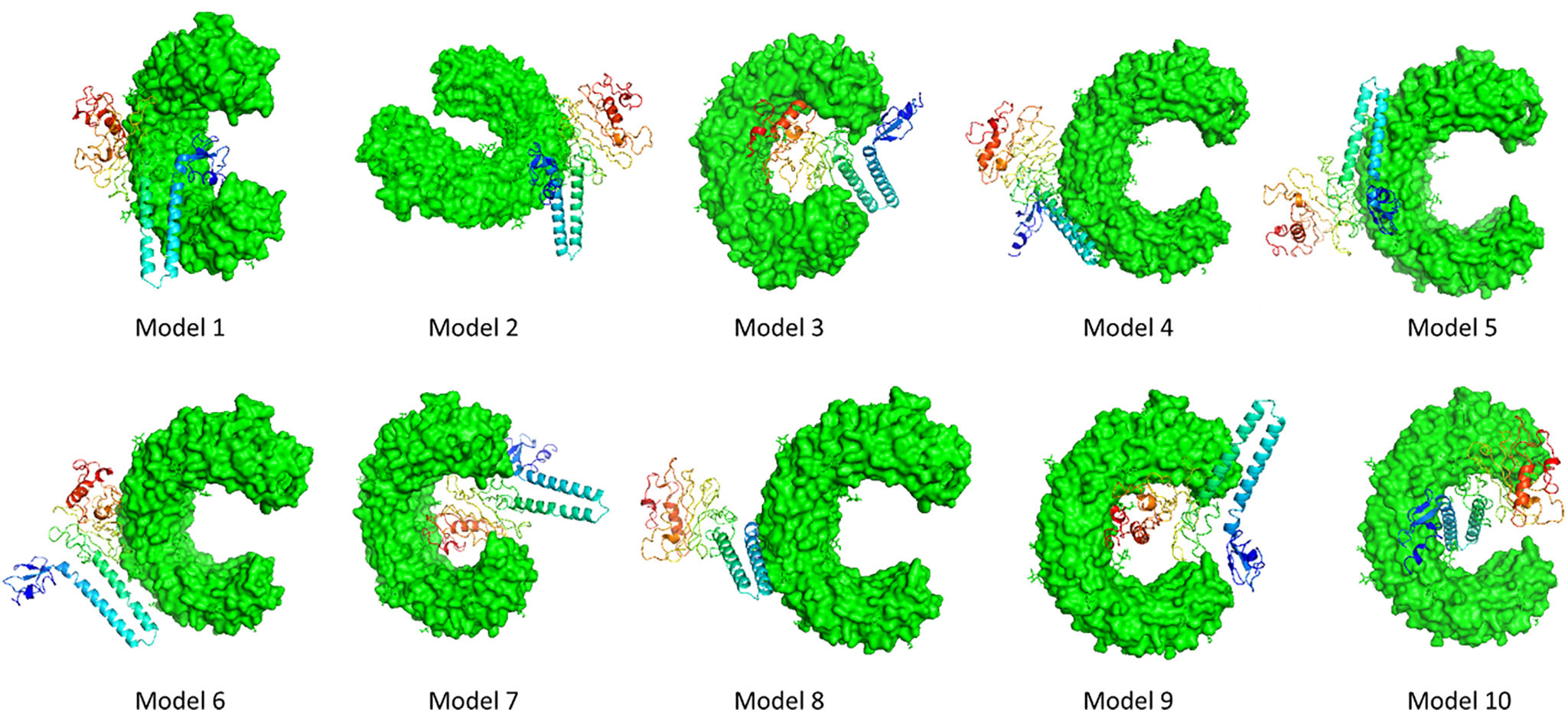
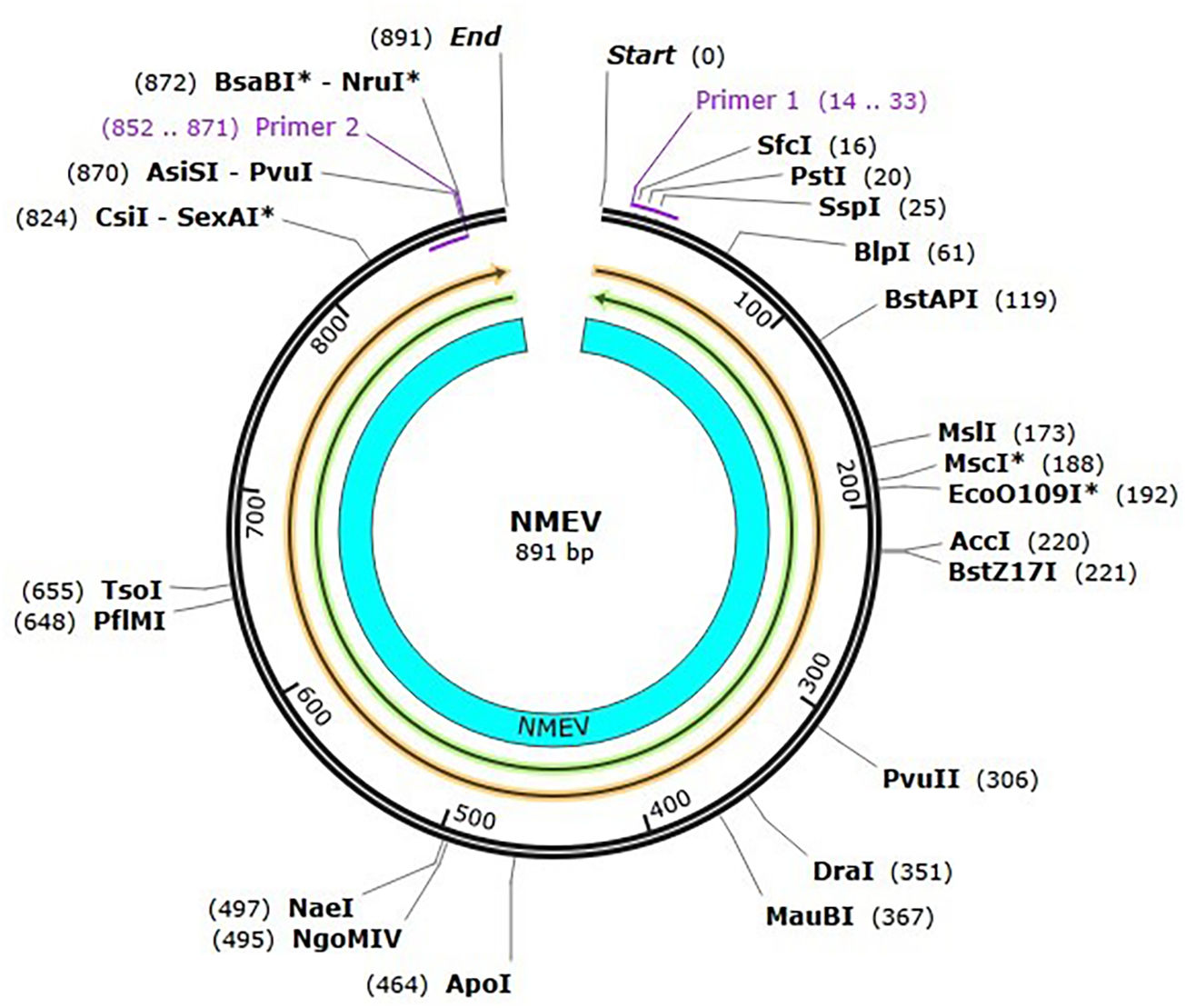
The development of a conventional vaccine against gonococci has been difficult since there are no precise correlates of immune protection and the mechanisms of protective immunity are not yet understood. However, the drop in gonorrhea infections might be attributed to the development of vaccines based on Neisseria meningitidis' outer membrane. The goal of this research was to develop a multi-epitope vaccination utilizing proteins from N. meningitidis and Neisseria gonorrhoeae. A Neisseria Multi-Epitope Vaccine (NMEV), containing Opa, ProA, ProB, RmpM, and BamD, was developed using immunophysicochemical informatics techniques. The vaccine consists of 297 amino acids. Antigenicity and sensitivity to NMEV were evaluated. NMEV generates a substantial amount of immune cells and cytokines. The total quality factor of the NMEV 2D structure is around 91%. The vaccine's safety, effectiveness, and other properties make it an attractive option for in vitro and in vivo testing. The suggested NMEV subunit vaccine has the potential to elicit a strong immune response, necessitating additional in vitro and in vivo studies to eliminate Neisseria infections.
El desarrollo de una vacuna convencional contra los gonococos ha sido difícil porque no existen correlatos precisos de la protección inmunitaria y aún no se comprenden los mecanismos de la inmunidad protectora. Sin embargo, la disminución de las infecciones por gonorrea podría atribuirse al desarrollo de vacunas basadas en la membrana externa de Neisseria meningitidis. El objetivo de esta investigación fue desarrollar una vacuna multiepítopo utilizando proteínas de N. meningitidis y N. gonorrhoeae. Se desarrolló una vacuna multiepítopo contra Neisseria (NMEV), que contiene Opa, ProA, ProB, RmpM y BamD, utilizando técnicas informáticas inmunofisicoquímicas. La vacuna consta de 297 aminoácidos. Se evaluaron la antigenicidad y la sensibilidad al NMEV. NMEV genera una cantidad sustancial de células inmunes y citocinas. El factor de calidad total de la estructura NMEV 2D ronda el 91%. La seguridad, eficacia y otras propiedades de la vacuna la convierten en una opción atractiva para pruebas in vitro e in vivo. La vacuna de subunidad NMEV sugerida tiene el potencial de provocar una fuerte respuesta inmune, lo que requiere estudios adicionales in vitro e in vivo para eliminar las infecciones por Neisseria.