Edited by: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
More infoCovaxin® is a COVID-19 vaccine created and produced by Bharat Biotech in India. The vaccine is based on the strain of SARS-CoV-2 that was first identified in India and has undergone Phase III clinical trials. Covaxin® has been authorized for emergency use in India and has been distributed as part of India's vaccination campaign. The vaccine has been shown to be effective in preventing COVID-19 infection and hospitalization, but additional studies are needed to determine its efficacy as well as safety in the long term.
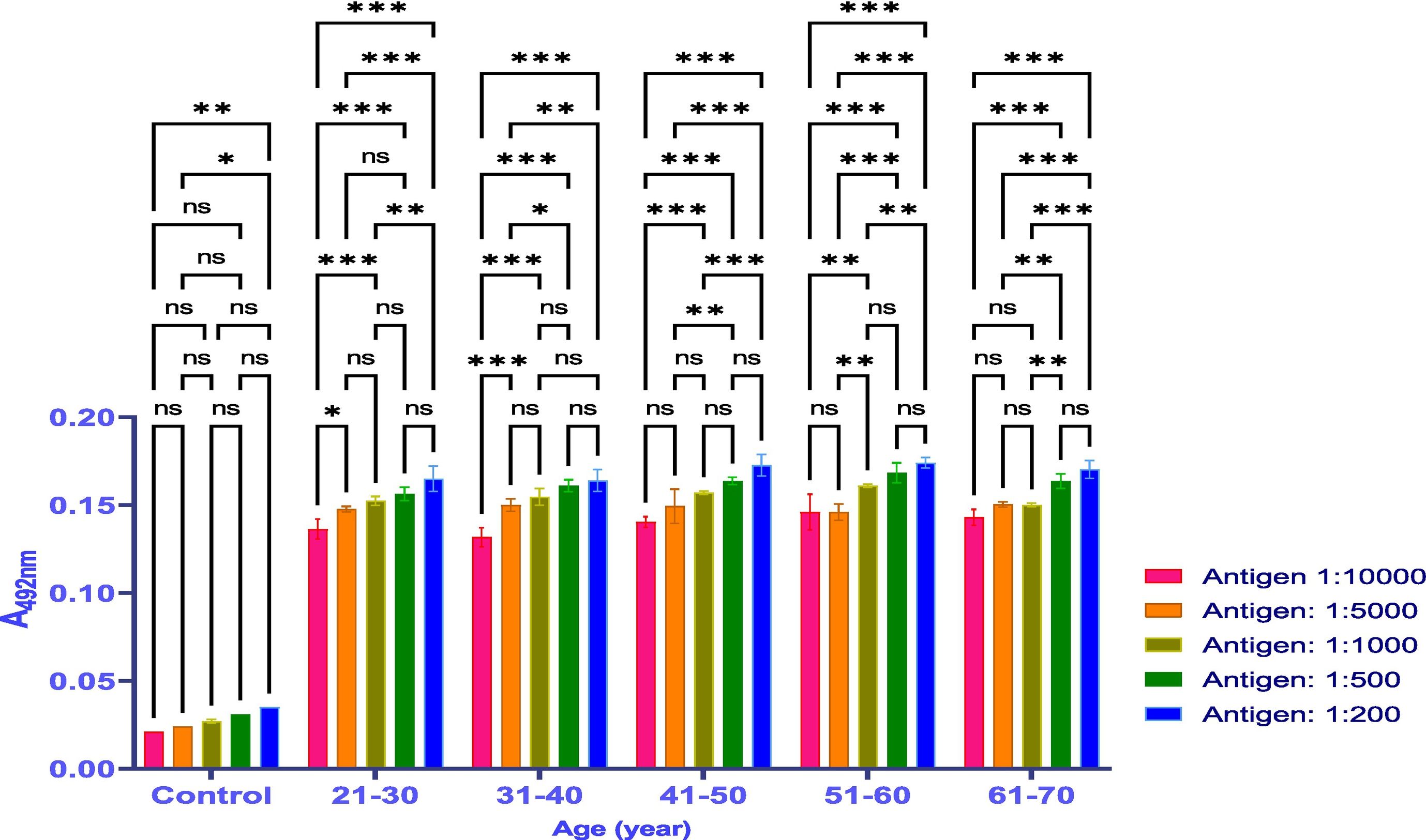
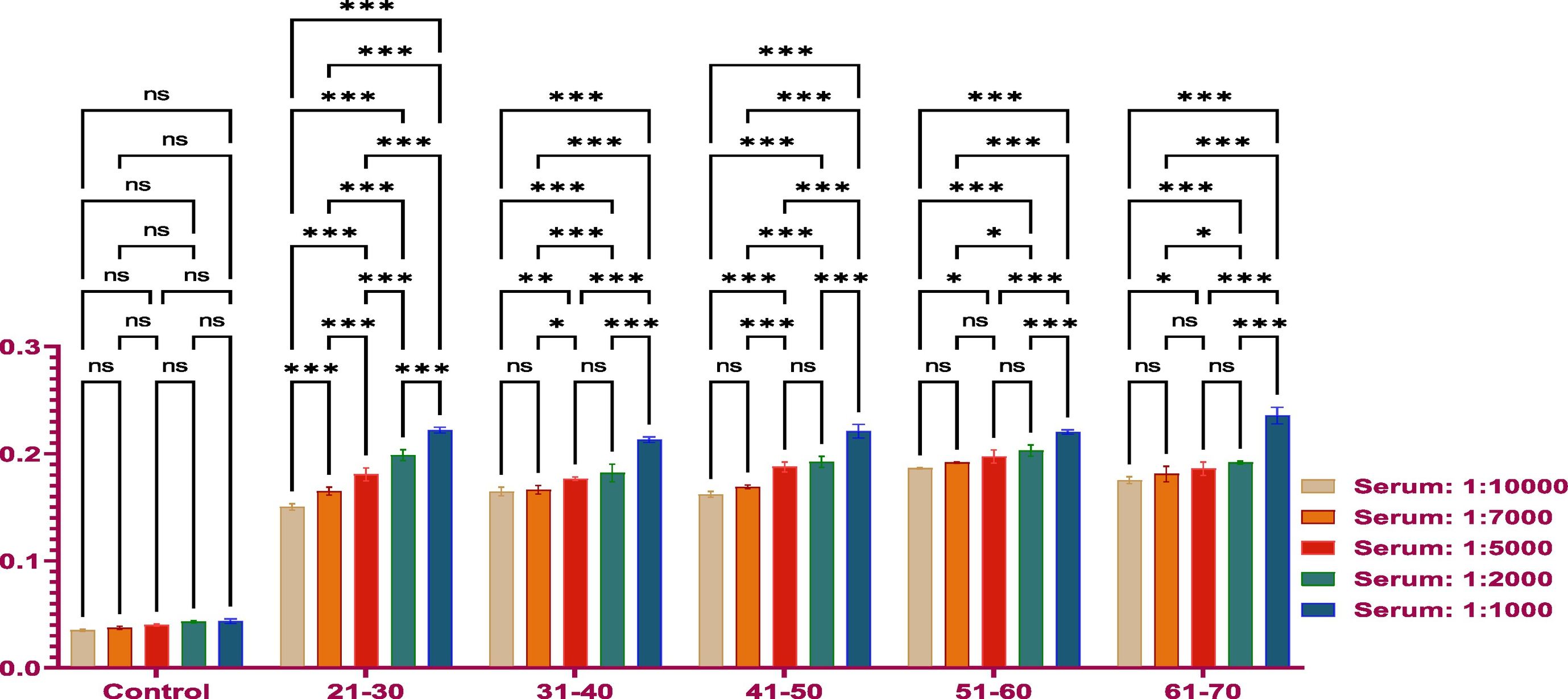
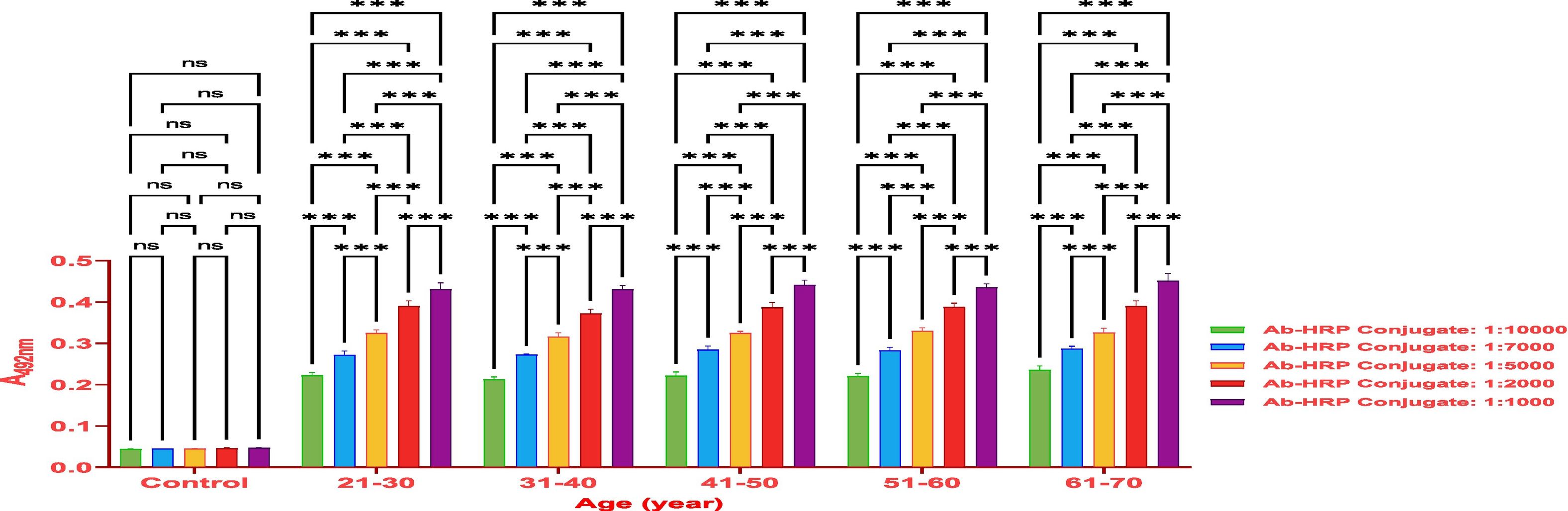
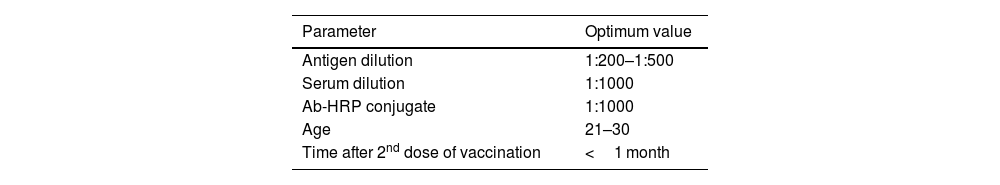
MethodsThe antibody titer against Covaxin® was detected through indirect ELISA immunoassay. Optimization was performed on 500 samples to get an idea and work further on a larger number of samples.
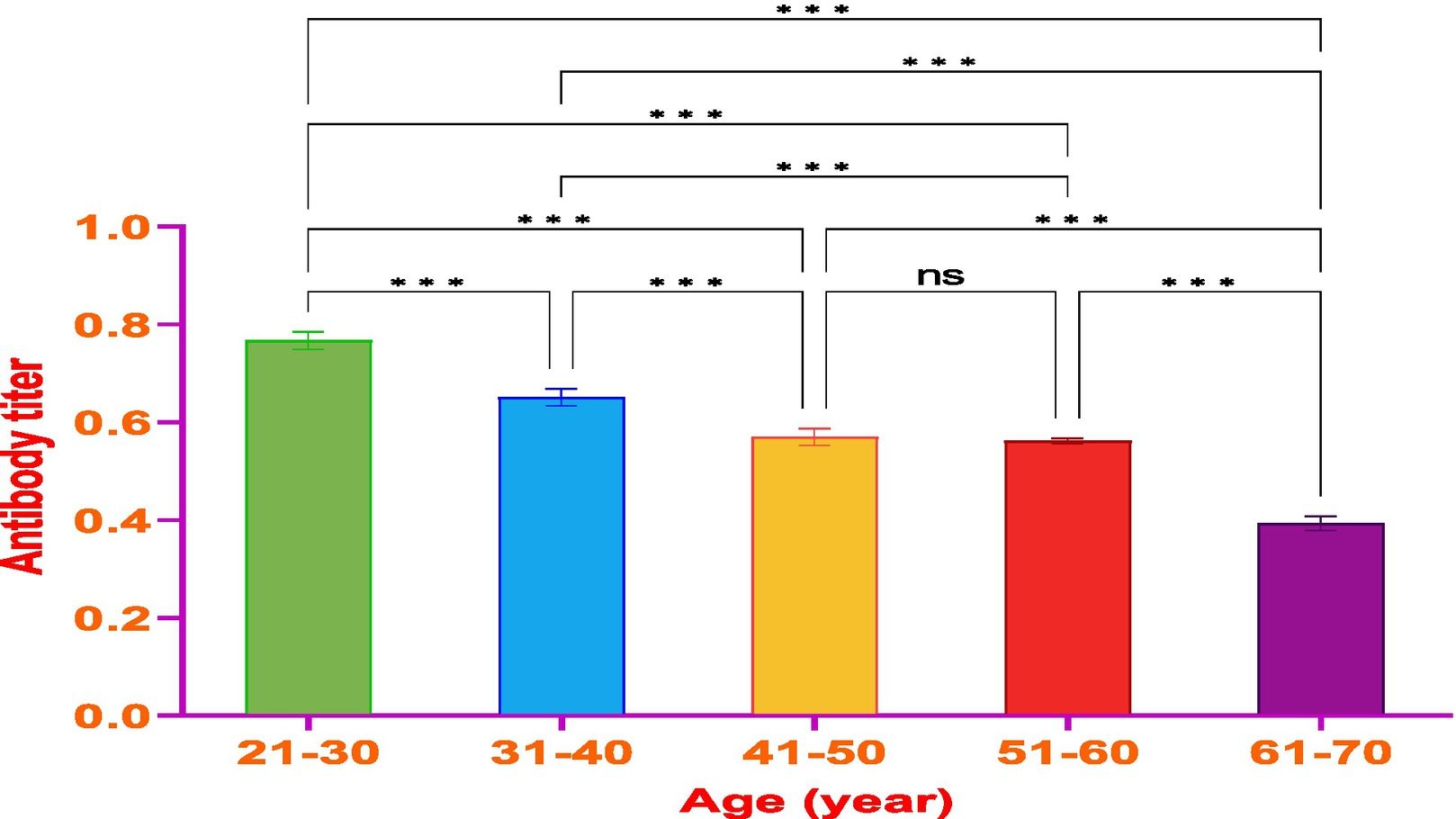
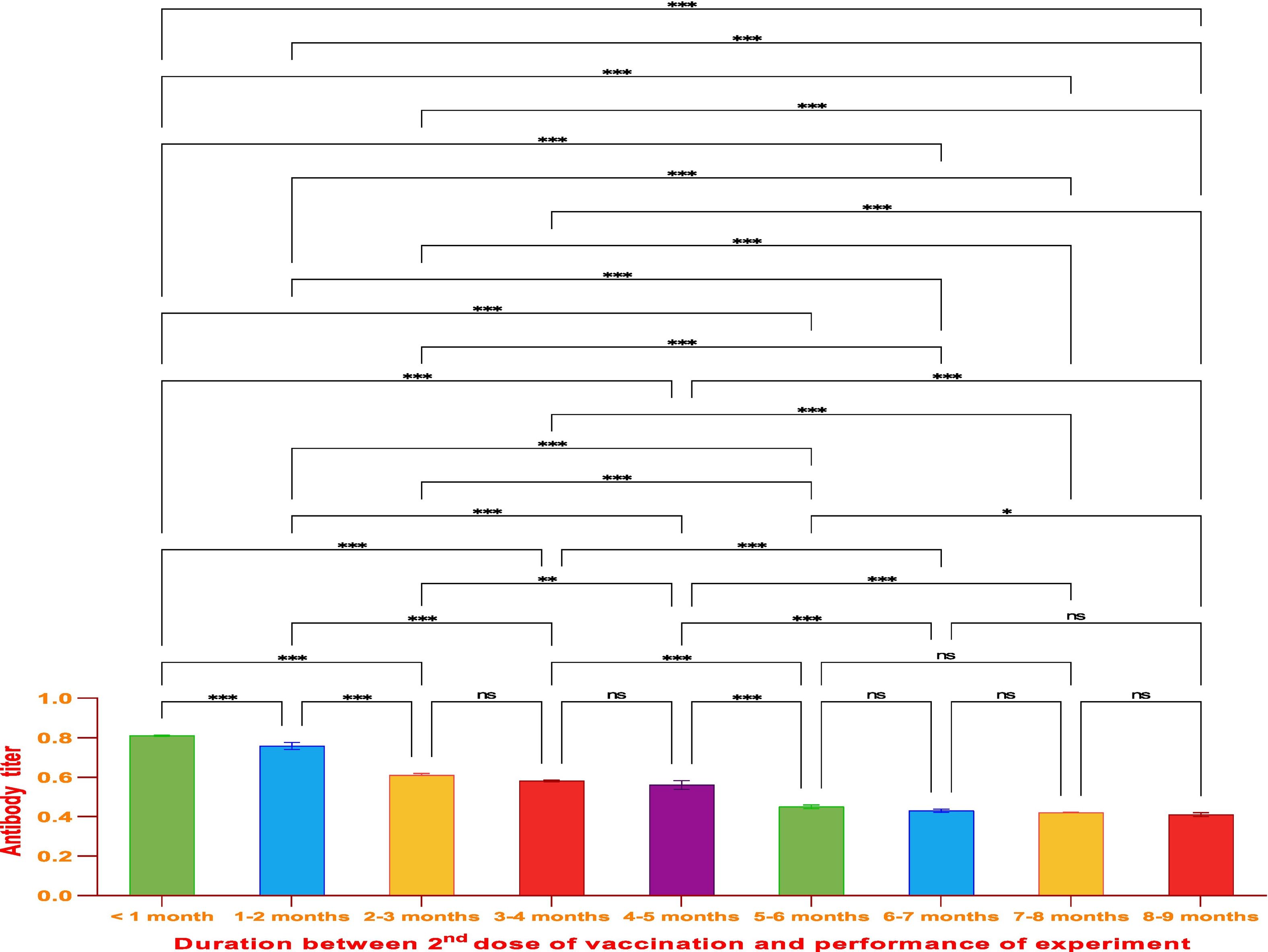
ResultsThe vaccine had the best immune response in individuals between the ages of 21 and 30 and the least response in those between 61 and 70. This was attributed to the phenomenon of immunosenescence, which explains the weakening of the immune system with age. Additionally, the study found that the equine anti-CoV-19 serum had a higher binding affinity with Covaxin®, highlighting the effectiveness of horse Ig against COVID-19 antigens.
ConclusionsThe study concluded that Covaxin® was effective in generating an immune response in individuals after 2 doses of vaccination; however, the generated immune response decreased with the time of vaccine administration and the age of the vaccinated. The study also showed that the ELISA technique used in this research is an efficient and sensitive method to evaluate vaccine efficiency and can be applied to a larger number of samples for further comparative analysis.
Covaxin® es una contra la COVID-19 creada y producida por Bharat Biotech en India. Dicha vacuna se basa en la cepa de SARS-CoV-2 inicialmente identificada en India, habiéndose sometido a los ensayos clínicos de Fase III. Covaxin® ha sido autorizada para uso urgente en India, habiéndose distribuido como parte de la campaña de vacunación en el país. Ha demostrado ser efectiva para la prevención de la infección y hospitalización por COVID-19, pero son necesarios estudios adicionales para determinar su eficacia, así como su seguridad a largo plazo.
MétodosEl título de anticuerpos contra Covaxin® fue detectado mediante inmunoensayo ELISA indirecto. La optimización fue realizada en 500 muestras, para adquirir una idea y trabajar adicionalmente en un número mayor de muestras.
ResultadosLa vacuna reflejó la mejor respuesta inmune en individuos de entre 21 y 30 años de edad, y la menor respuesta en personas de entre 61 y 70 años. Esto se atribuyó al fenómeno de inmunosenescencia, que explica el debilitamiento del sistema inmune con la edad. Además, el estudio encontró que el suero equino anti-CoV-19 tenía una mayor afinidad de unión a Covaxin®, lo cual subraya la efectividad del Ig equino contra los antígenos de COVID-19.
ConclusionesEl estudio concluyó que Covaxin® fue efectiva para generar una respuesta inmune en los individuos, tras la administración de dos dosis de vacunación. Sin embargo, la respuesta inmune generada disminuyó con el tiempo transcurrido desde la administración de la vacuna y la edad de las personas vacunadas. El estudio reflejó también que la técnica ELISA utilizada en esta investigación fue un método eficiente y sensible para evaluar la eficiencia vacunal, pudiendo aplicarse a un número de muestras mayor, de cara a un análisis comparativo adicional.