to analyze the factors associated with the non-development of immunogenicity in the adult population vaccinated against HBV.
Materials and MethodsA case-control study was performed in a population of patients vaccinated against HBV, who were titrated to antibodies against HBV surface antigen between the years 2015 and 2016. Adults (>18 years) vaccinated against HBV, titrated to anti-HBs < 10 mIU/mL, and who did not present chronic hepatitis B or previous history of HBV infection were defined as cases. The controls were defined with the same characteristics of the cases, the only difference being a titre of anti-HBs ≥ 10 mIU/mL. The factors analyzed were age, sex, number of doses, and some comorbidities such as HIV infection, type 2 diabetes mellitus, and chronic kidney disease on hemodialysis. A bivariate analysis was performed using the Mann-Whitney Chi2 and U tests, as well as a multivariate analysis using binary logistic regression to generate a predictive model.
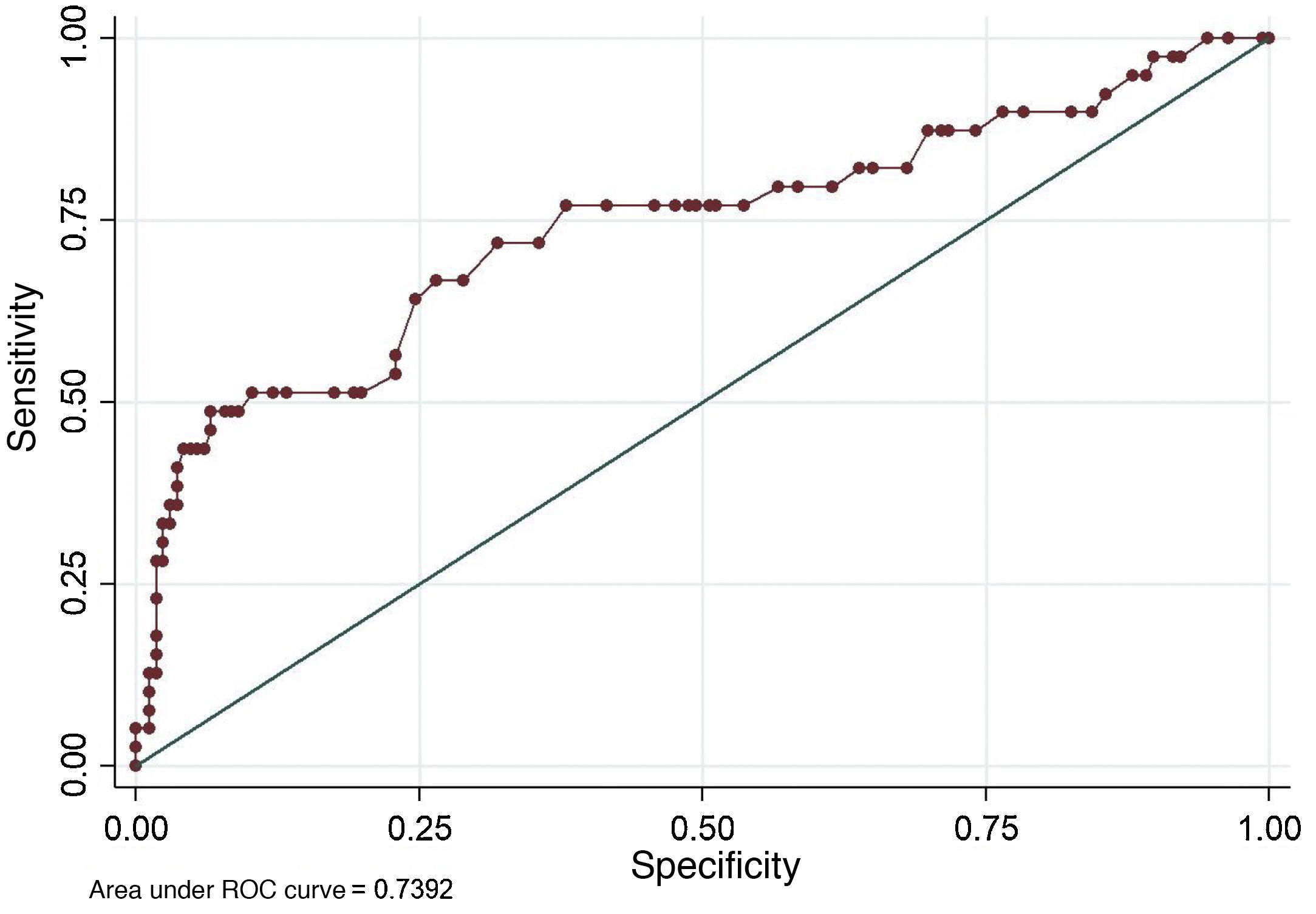
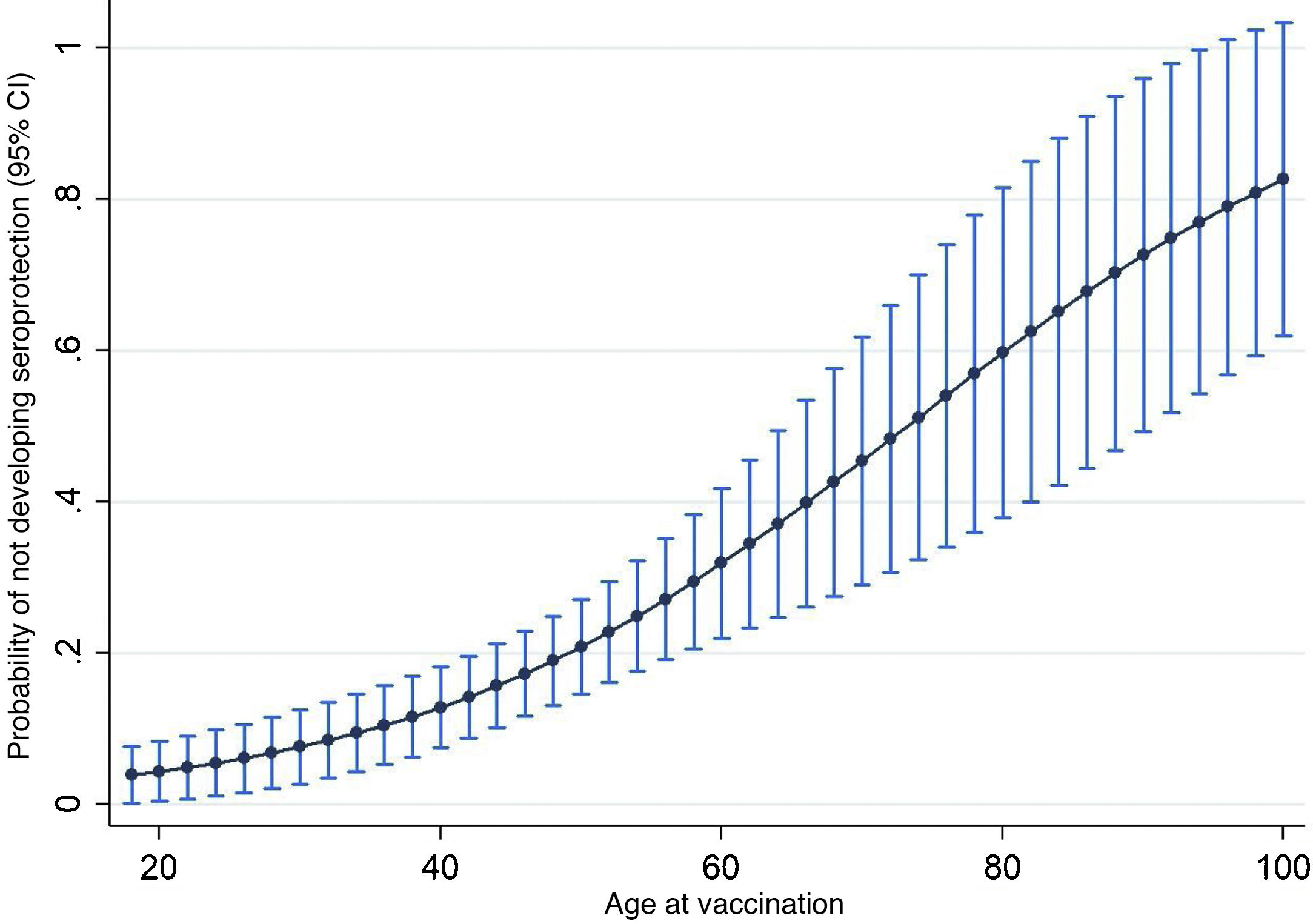
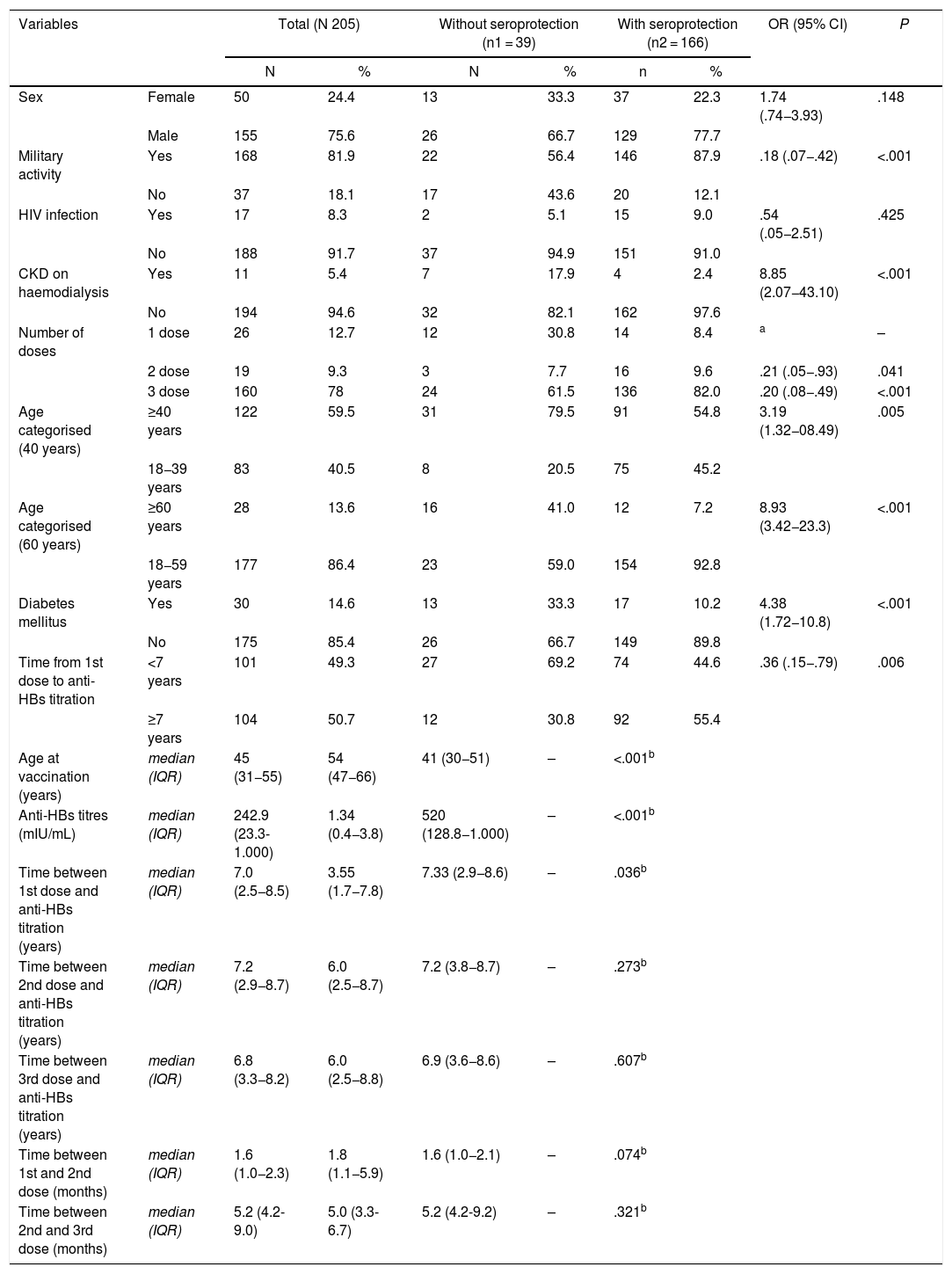
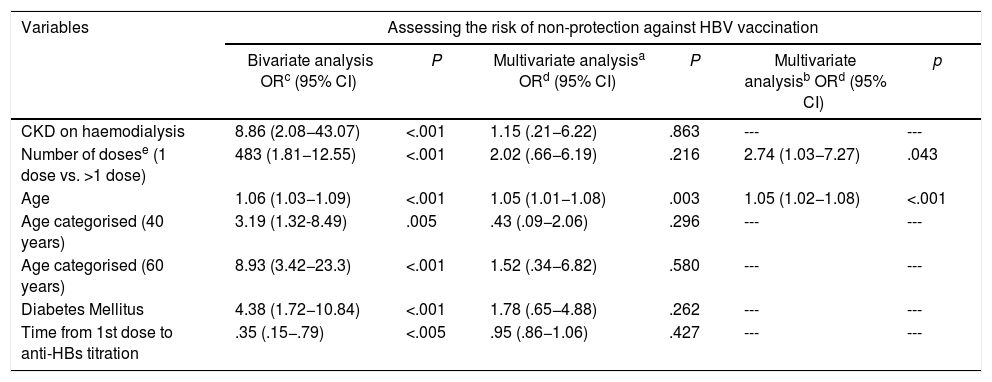
ResultsThe cohort of patients with anti-HBs titers was composed of 205 participants, which included 39 cases and 166 controls (power: 91%, significance of 0.05). The bivariate analysis showed that the variables age (OR 3.19, P = .005), number of vaccine doses (OR 4.82, P = .001), hemodialysis CKD (OR 8.85, P = .001), Diabetes mellitus 2(OR 4.38, P = .001), and the time from onset of vaccination (OR 0.36, P = .006), were associated with non-development of seroprotection against HBV. Likewise, age groups >40 years and >60 years were highly significant in this analysis, OR 3.19 and OR 8.93, respectively. In the multivariate analysis, only age as a numerical variable (OR: 1.05) and number of doses (OR: 2.74), after being adjusted by the other variables, were the only ones that explained the non-development of seroprotection in adults. The final logistic model with the variables indicated explains in 74% the non-seroprotection.
ConclusionsAge and number of doses are the only associated variables able to predict with high certainty the non-seroprotective vaccination against HBV.
Analizar los factores asociados al no desarrollo de seroprotección en población adulta vacunada contra VHB.
Materiales y MétodosSe realizó un estudio de casos y controles en una muestra de pacientes adultos vacunados contra VHB, a quienes se les midió títulos de anticuerpos contra el antígeno de superficie para VHB (anti-HBs) entre los años 2015 y 2016. Se definió como caso a los adultos (≥18 años) vacunados contra el VHB, con titulación de anti-HBs < 10 mUI/mL, y que no haya presentado hepatitis B crónica o antecedente de infección resuelta por el VHB. Los controles fueron definidos con las mismas características de los casos, siendo la única diferencia una titulación de anti-HBs ≥ 10 mUI/mL. Los factores analizados fueron la edad, el sexo, el número de dosis y algunas comorbilidades como infección por VIH, Diabetes mellitus tipo 2 y enfermedad renal crónica en hemodiálisis. Se realizó un análisis bivariante mediante las pruebas de Chi2 y U de Mann-Whitney, así como un análisis multivariado mediante regresión logística binaria para generar un modelo predictivo.
ResultadosLa cohorte de pacientes con titulación de anti-HBs estuvo formada por 205 participantes, la cual incluyó 39 casos y 166 controles (potencia: 91%, significancia α de 0.05). El análisis bivariante mostró que las variables edad (OR 3.19; P = .005), número de dosis vacunal (OR 4.82; P = .001), ERC en hemodiálisis (OR 8.85; P = .001), Diabetes mellitus (OR4.38; P = .001), y el tiempo desde el inicio de la vacunación (OR 0.36; P = .006), se asociaron al no desarrollo de seroprotección contra el VHB. Asimismo, los grupos de edad ≥40 años y ≥60 años, resultaron altamente significativos en este análisis, OR 3.19 y OR 8.93 respectivamente. En el análisis multivariado, solo la edad como variable numérica (OR: 1.05) y el número de dosis (OR: 2.74), tras ser ajustadas por las demás variables, fueron las únicas que explicaron el no desarrollo de seroprotección en adultos. El modelo logístico final con las variables señaladas explica en un 74% la no seroprotección.
ConclusionesLa edad y el número de dosis son las únicas variables asociadas capaces de predecir con alta certeza la no seroprotección vacunal contra el VHB.
Artículo
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora