Certain vaccine-preventable diseases such as invasive pneumococcal disease (IPD), pneumonia, and otitis media (OM) are caused by Streptococcus pneumoniae. Pneumococcal conjugate vaccines (PCVs) have significantly contributed to reduce the burden of pneumococcal disease. The evidence from published literature indicates that the pneumococcal Haemophilus influenzae protein D conjugate vaccine and the 13-valent PCV have similar impact against pneumococcal disease burden. This opinion has been adopted by independent recognised public health entities such as the World Health Organization and the Pan American Health Organization. The net effect is limited by the continuous replacement of serotypes not contained in the formulations. The studies focusing on specific serotypes should recognise that the main goal of the vaccination with PCVs is to reduce the overall burden of disease regardless the serotypes already contained into formulation.
Ciertas enfermedades prevenibles con vacunas, como la enfermedad neumocócica invasiva (ENI), la neumonía y la otitis media (OM) son causadas por Streptococcus pneumoniae. Las vacunas neumocócicas conjugadas (VNC) han contribuido de manera considerable a reducir la carga de la enfermedad neumocócica. La evidencia bibliográfica publicada indica que la vacuna neumocócica conjugada con la proteína D de Haemophilus influenzae y la VNC de 13 valencias tienen un impacto comparable sobre la carga de la enfermedad neumocócica. Esta opinión ha sido emitida por entidades independientes y reconocidas de salud pública como la Organización Mundial de la Salud (OMS) y la Organización Panamericana de la Salud (OPS). El efecto neto se ve limitado por el reemplazo continuo de serotipos no incluidos en las formulaciones. Los estudios centrados en serotipos concretos deberían reconocer que el objetivo principal de la vacunación con las VNC es reducir la carga global de la enfermedad, independientemente de los serotipos contenidos en la formulación.
The bacteria Streptococcus pneumoniae (S. pneumoniae) causes a number of invasive diseases such as meningitis, bacteraemia, sepsis, bacterial pneumonia and non-invasive mucosal diseases, such as pneumonia without bacteraemia, otitis media (OM) and sinusitis.1–3 This pathogen mainly colonises the nasopharynx of infants and young children and is transmitted by respiratory droplets.1,3 More than 90 serotypes have been described to date, of which 6–11 are considered responsible for more than 70% of invasive pneumococcal disease (IPD).1
IPD is a public health burden, with higher incidence and mortality rates in children <2 and adults >65 years.1,4,5 In 2010, the overall incidence of IPD in Europe was 5.2/100,000, with 18.5 cases per 100,000 children<1 year.4 The incidence of IPD in developing countries is even higher, with an estimated annual incidence of IPD in African children of 62.6/100,000.6
S. pneumoniae is the main bacterium responsible for community-acquired pneumonia (CAP), which often results in hospital admissions and surgery. It is also responsible for 78% of lobar pneumonia in children.1 In 2015, it was estimated that approximately 8.9 million episodes of pneumonia in children =5 years of age had occurred, of which 3.5 million were considered severe.7 Of the 257,000 estimated deaths worldwide, the highest proportion occurred in Asia and Africa.7
More than 80% of children <3 years suffer at least one episode of acute OM (AOM) and 40% experience more than 6 episodes before their 7th birthday.8 Although less severe than other pneumococcal diseases, AOM disrupts the daily activities of children and their families, and is responsible for significant health expenditure and antibiotic consumption.9
There are currently 2 types of anti-pneumococcal vaccines: polysaccharide and conjugate. The former, available since the early 1980s, contain 23 pneumococcal serotypes (polysaccharides) (VNP23). However, they provide little immunogenicity in children<2 and do not produce an anamnestic response at any age.1
Binding S. pneumoniae polysaccharides with an immunogenic carrier resulted in the current conjugate vaccines,1 which enhance the immune response, especially in children <2 years of age, and induce immune memory.1 Of all the pneumococcal conjugate vaccines (PCV), the 7-valent vaccine (PCV7), which included 7 pneumococcal polysaccharides (4, 6B, 9V, 14, 18C, 19F and 23F) conjugated with a non-toxic variant of the diphtheria toxoid cross-reacting material 197 (CRM197), was the first to be introduced in immunisation programmes.10 PCV7 was gradually replaced by the new 10 and 13-valent conjugate vaccines.1
The PCV with Protein D of Haemophilus influenzae (H. influenzae) (Haemophilus influenzae protein D conjugate vaccine (PHiD-CV); Synflorix; GSK, Belgium), contains 10 pneumococcal polysaccharides (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F), 8 of which are conjugated separately to the non-typeable H. influenzae-derived protein D.1,10 The other 2 are conjugated to the tetanus (18C) and diphtheria (19F) toxoids. PHiD-CV is currently indicated for immunisation against IPD, pneumonia and AOM caused by S. pneumoniae in Infants and children<5 years.1
The 13-valent PCV (PCV13) contains 13 pneumococcal polysaccharides (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) individually conjugated to CRM197 (PVC13; Prevenar 13; Pfizer, U.S.A.).1,10 Its indications are immunisation against IPD, pneumonia and AOM caused by S. pneumoniae in infants, children, adolescents and adults.1
The World Health Organization (WHO) recommends the inclusion of PHiD-CV and PCV13 in annual childhood immunisation schedules.1 Since 2009, many countries have introduced them into their childhood vaccination programmes. Hence the need for comparative data on the impact of both vaccinations in different populations at different times, in decision-making on vaccination programmes.
The parameters for assessing the performance of a vaccine should consider the context in which the vaccination strategy is implemented. Therefore, we define efficacy as health outcomes evaluated under ideal application conditions as measured by controlled clinical trials,11 we define effectiveness as health outcomes achieved under real application conditions of the vaccine as measured by epidemiological, analytical, observational, case-control studies, prospective cohorts or indirect cohorts and the impact of health outcomes on a vaccinated population in a given geographical area measured by descriptive before-and-after trend studies11 (non-analytical observational studies of sufficiently large cohorts with a sufficiently long time horizon will be considered impact studies for the purposes of this article).
There is a limited amount of comparative data on the impact of PHiD-CV or PCV13. Therefore, it is difficult to evaluate the complexities of vaccine effectiveness and impact based on isolated cases and serotypes alone.10 The objectives of this study were to put the data on the impact of the currently available PCVs into context, and to review the position of international bodies regarding the conclusions and recommendations on the use of PCVs in order to reduce the burden of pneumococcal disease.
Material and methodsIn drafting this manuscript, a non-systemic narrative review of the literature was conducted through the Medline database via PubMed to identify articles evaluating the impact of PHiD-CV and PCV13 in children =18 years published from January 1, 2013 to February 28, 2019 (date of the last search), combining the following terms: pneumococcal conjugate vaccine, PCV13, 13-valent pneumococcal conjugate vaccine, PCV10, 10-valent pneumococcal conjugate vaccine, PHiD-CV, 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine, invasive pneumococcal disease, invasive disease, pneumonia, community-acquired pneumonia, otitis media, acute otitis media. The articles yielded from this search are shown in Tables 1–4. Eligible articles included publications on laboratory-based surveillance studies before and after use of the vaccine, time-series studies and prospective or retrospective population-based observational cohort studies. To compare some final results for which impact studies are not available, case-control and cohort studies assessing effectiveness, not impact, were used as impact “proxies”. We excluded publications reporting pilot studies, protocols, preclinical studies and cost-effectiveness analyses, nasopharyngeal carriage, studies in animal models and studies whose objective was vaccination in the adult population. Letters to the editor, editorials, comments, opinions and articles evaluating vaccine coverage alone were also excluded. Other technical or scientific documents were obtained through the Google search engine.
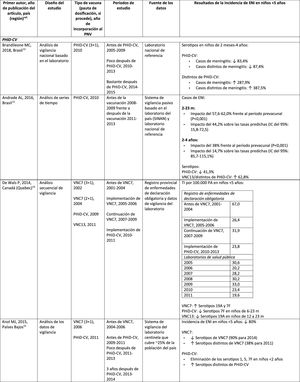
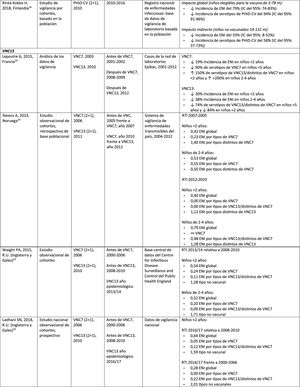
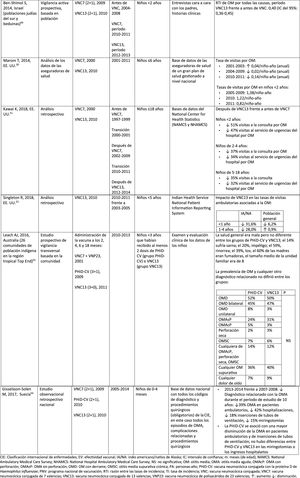
Impact PHiD-CV and PCV VNC13 on the incidence of IPD in children <5 years.
CDC: U.S. Centres for Disease Control and Prevention; U.S.A.: United States; IPD: invasive pneumococcal disease; VE: vaccine effectiveness; CI: confidence interval; m: months (of age); PY: person-year; PHiD-CV: Haemophilus influenzae protein D conjugate vaccine; NVP: national vaccination programme; ref.: reference; IRR: incidence rate ratio; U.K.: United Kingdom; SINAN: Brazilian Notifiable Diseases Information System; IR: incidence rate; PCV: pneumococcal conjugate vaccine; PVC7: 7-valent pneumococcal conjugate vaccine; PVC13: 13-valent pneumococcal conjugate vaccine; ?: increase; ?: decrease.
a Selected counties in California, Colorado, Georgia, Maryland, New York, Oregon, Tennessee and the states of Connecticut, Minnesota, Nuevo Mexico.
b A.P. Bolzano, A.P. Trento, Emilia-Romagna, Friuli-Venezia Giulia, Lombardy, Piemonte, Veneto.
c Very seldom used in Spain.28
Impact of PHiD-CV and PVC13 on pneumonia.
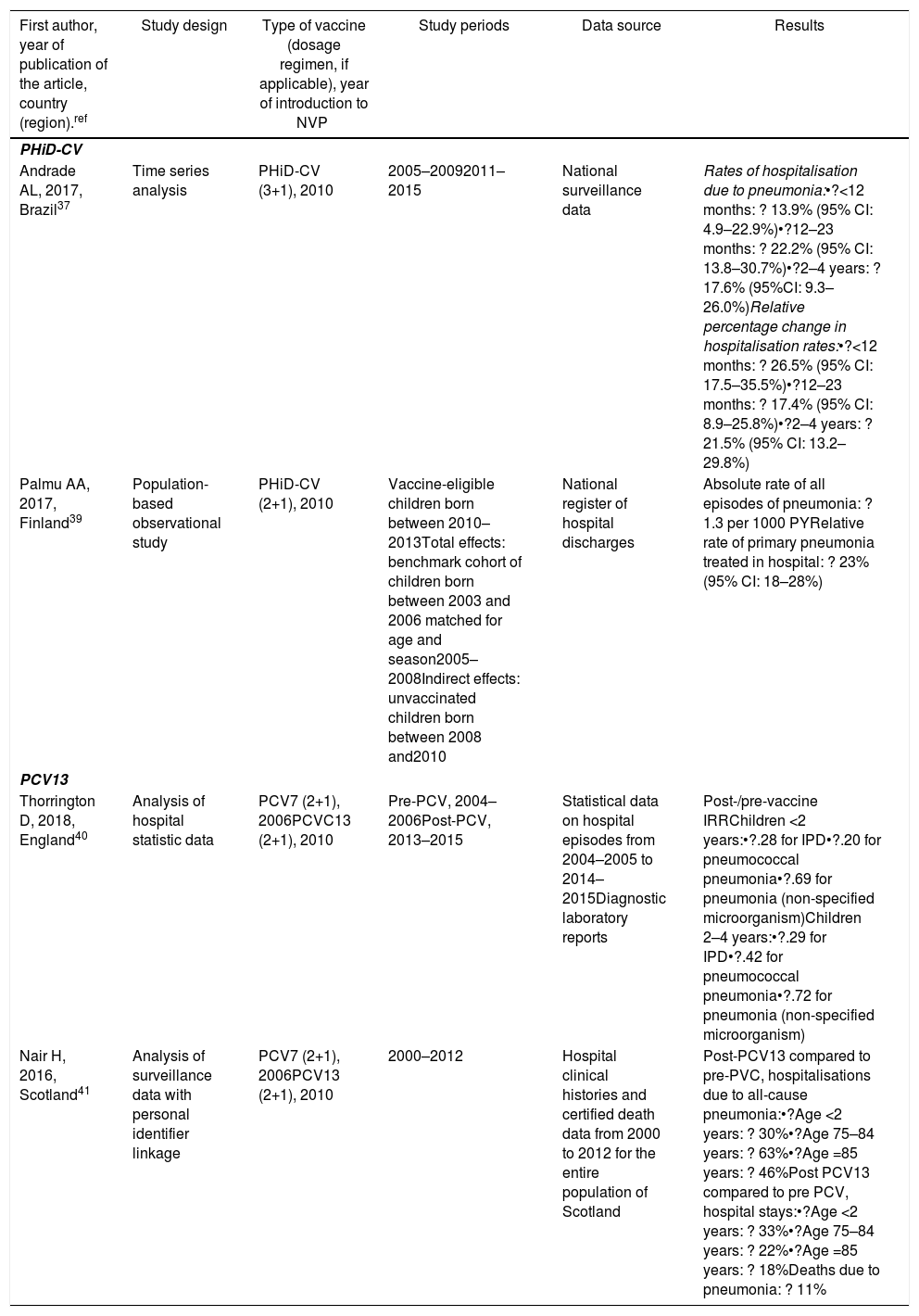
| First author, year of publication of the article, country (region).ref | Study design | Type of vaccine (dosage regimen, if applicable), year of introduction to NVP | Study periods | Data source | Results |
|---|---|---|---|---|---|
| PHiD-CV | |||||
| Andrade AL, 2017, Brazil37 | Time series analysis | PHiD-CV (3+1), 2010 | 2005–20092011–2015 | National surveillance data | Rates of hospitalisation due to pneumonia:•?<12 months: ? 13.9% (95% CI: 4.9–22.9%)•?12–23 months: ? 22.2% (95% CI: 13.8–30.7%)•?2–4 years: ? 17.6% (95%CI: 9.3–26.0%)Relative percentage change in hospitalisation rates:•?<12 months: ? 26.5% (95% CI: 17.5–35.5%)•?12–23 months: ? 17.4% (95% CI: 8.9–25.8%)•?2–4 years: ? 21.5% (95% CI: 13.2–29.8%) |
| Palmu AA, 2017, Finland39 | Population-based observational study | PHiD-CV (2+1), 2010 | Vaccine-eligible children born between 2010–2013Total effects: benchmark cohort of children born between 2003 and 2006 matched for age and season2005–2008Indirect effects: unvaccinated children born between 2008 and2010 | National register of hospital discharges | Absolute rate of all episodes of pneumonia: ? 1.3 per 1000 PYRelative rate of primary pneumonia treated in hospital: ? 23% (95% CI: 18–28%) |
| PCV13 | |||||
| Thorrington D, 2018, England40 | Analysis of hospital statistic data | PCV7 (2+1), 2006PCVC13 (2+1), 2010 | Pre-PCV, 2004–2006Post-PCV, 2013–2015 | Statistical data on hospital episodes from 2004–2005 to 2014–2015Diagnostic laboratory reports | Post-/pre-vaccine IRRChildren <2 years:•?.28 for IPD•?.20 for pneumococcal pneumonia•?.69 for pneumonia (non-specified microorganism)Children 2–4 years:•?.29 for IPD•?.42 for pneumococcal pneumonia•?.72 for pneumonia (non-specified microorganism) |
| Nair H, 2016, Scotland41 | Analysis of surveillance data with personal identifier linkage | PCV7 (2+1), 2006PCV13 (2+1), 2010 | 2000–2012 | Hospital clinical histories and certified death data from 2000 to 2012 for the entire population of Scotland | Post-PCV13 compared to pre-PVC, hospitalisations due to all-cause pneumonia:•?Age <2 years: ? 30%•?Age 75–84 years: ? 63%•?Age =85 years: ? 46%Post PCV13 compared to pre PCV, hospital stays:•?Age <2 years: ? 33%•?Age 75–84 years: ? 22%•?Age =85 years: ? 18%Deaths due to pneumonia: ? 11% |
IPD: invasive pneumococcal disease; CI: confidence interval; PY: person-year; PHiD-CV: Haemophilus influenzae protein D conjugate vaccine; NVP: national vaccination programme; IRR: incidence rate ratio; PCV: Pneumococcal conjugate vaccine; PCV7: 7-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine; ?: increase ?: decrease.
Impact of PHiD-CV and PCV13 on AOM.
ICD: International classification of diseases; VE: vaccine effectiveness; AI/AN: American Indian/Alaska native; CI: confidence interval; m: months (of age); NAMCS: National Ambulatory Medical Care Survey; NHAMCS: National Hospital Ambulatory Medical Care Survey; NS: not significant; OM: otitis media; AOM: acute otitis media; AOMwP: AOMA with perforation; AOMwoP: AOM without perforation; OME: OM with effusion; CSOM: chronic suppurative otitis media; PY: person-year; PHiD-CV: Haemophilus influenzae protein D conjugate vaccine; NVP: national vaccination programme; IRR: incident rate ratio; IR: incident rate; PCV: pneumococcal conjugate vaccine; PCV7: 7-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine; PCV23: 23-valent pneumococcal polysaccharide vaccine; ?: increase ?: decrease.
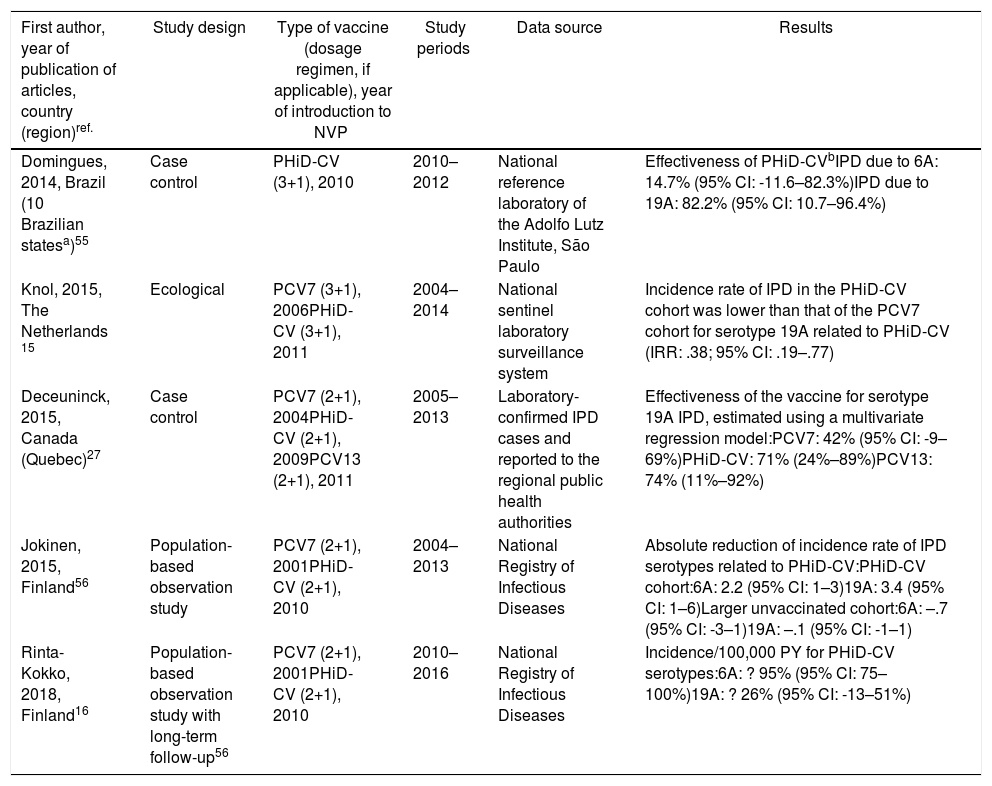
Cross-protection of PHiD-CV against 19A and 6A serotypes in vaccinated children.
| First author, year of publication of articles, country (region)ref. | Study design | Type of vaccine (dosage regimen, if applicable), year of introduction to NVP | Study periods | Data source | Results |
|---|---|---|---|---|---|
| Domingues, 2014, Brazil (10 Brazilian statesa)55 | Case control | PHiD-CV (3+1), 2010 | 2010–2012 | National reference laboratory of the Adolfo Lutz Institute, São Paulo | Effectiveness of PHiD-CVbIPD due to 6A: 14.7% (95% CI: -11.6–82.3%)IPD due to 19A: 82.2% (95% CI: 10.7–96.4%) |
| Knol, 2015, The Netherlands 15 | Ecological | PCV7 (3+1), 2006PHiD-CV (3+1), 2011 | 2004–2014 | National sentinel laboratory surveillance system | Incidence rate of IPD in the PHiD-CV cohort was lower than that of the PCV7 cohort for serotype 19A related to PHiD-CV (IRR: .38; 95% CI: .19–.77) |
| Deceuninck, 2015, Canada (Quebec)27 | Case control | PCV7 (2+1), 2004PHiD-CV (2+1), 2009PCV13 (2+1), 2011 | 2005–2013 | Laboratory-confirmed IPD cases and reported to the regional public health authorities | Effectiveness of the vaccine for serotype 19A IPD, estimated using a multivariate regression model:PCV7: 42% (95% CI: -9–69%)PHiD-CV: 71% (24%–89%)PCV13: 74% (11%–92%) |
| Jokinen, 2015, Finland56 | Population-based observation study | PCV7 (2+1), 2001PHiD-CV (2+1), 2010 | 2004–2013 | National Registry of Infectious Diseases | Absolute reduction of incidence rate of IPD serotypes related to PHiD-CV:PHiD-CV cohort:6A: 2.2 (95% CI: 1–3)19A: 3.4 (95% CI: 1–6)Larger unvaccinated cohort:6A: –.7 (95% CI: -3–1)19A: –.1 (95% CI: -1–1) |
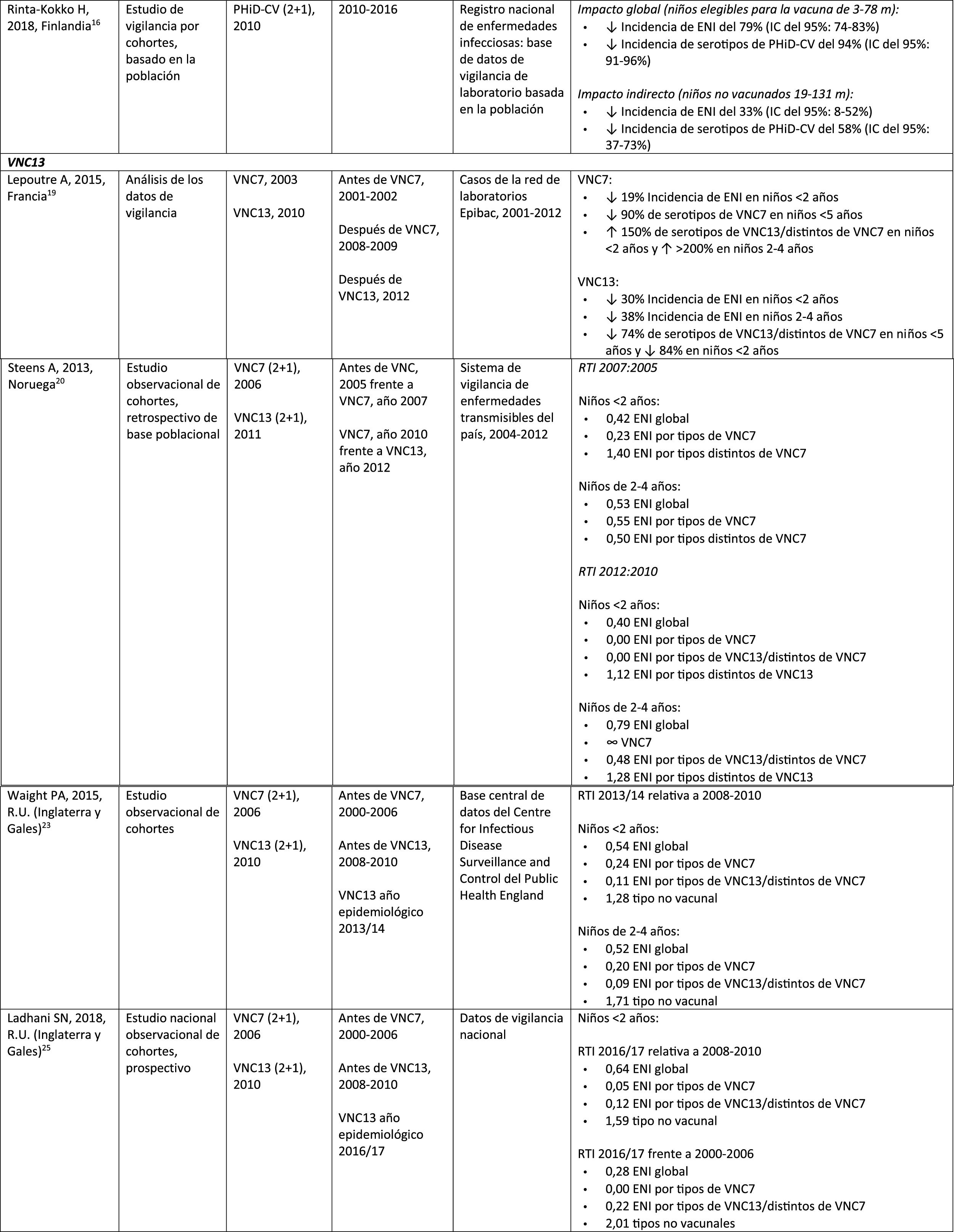
| Rinta-Kokko, 2018, Finland16 | Population-based observation study with long-term follow-up56 | PCV7 (2+1), 2001PHiD-CV (2+1), 2010 | 2010–2016 | National Registry of Infectious Diseases | Incidence/100,000 PY for PHiD-CV serotypes:6A: ? 95% (95% CI: 75–100%)19A: ? 26% (95% CI: -13–51%) |
IPD: invasive pneumococcal disease; CI: confidence interval; m: months (of age); PY: person-year; PHiD-CV Haemophilus influenzae protein D conjugate vaccine; IRR: incident rate ratio; PCV: pneumococcal conjugate vaccine; PCV7: 7-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine.
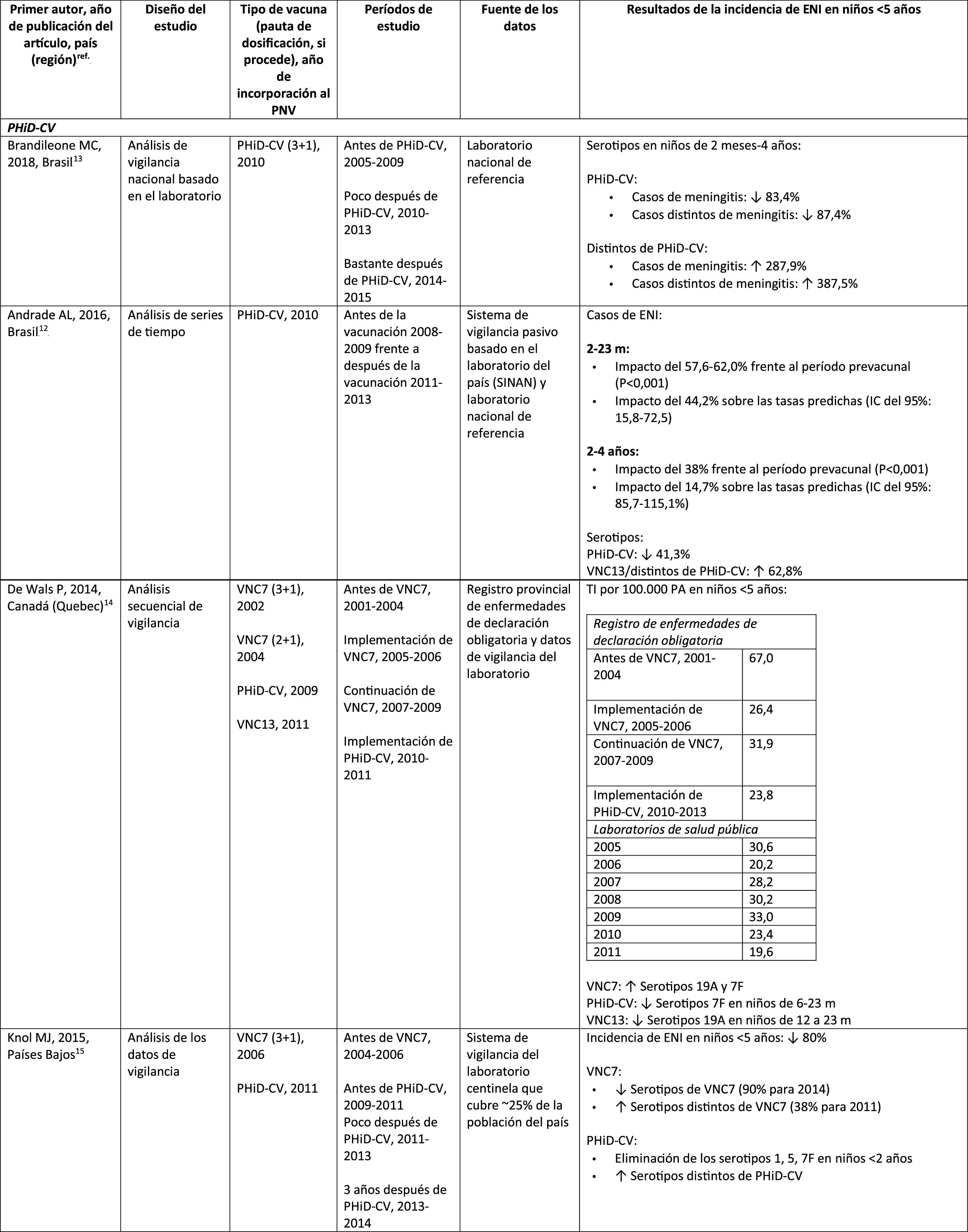
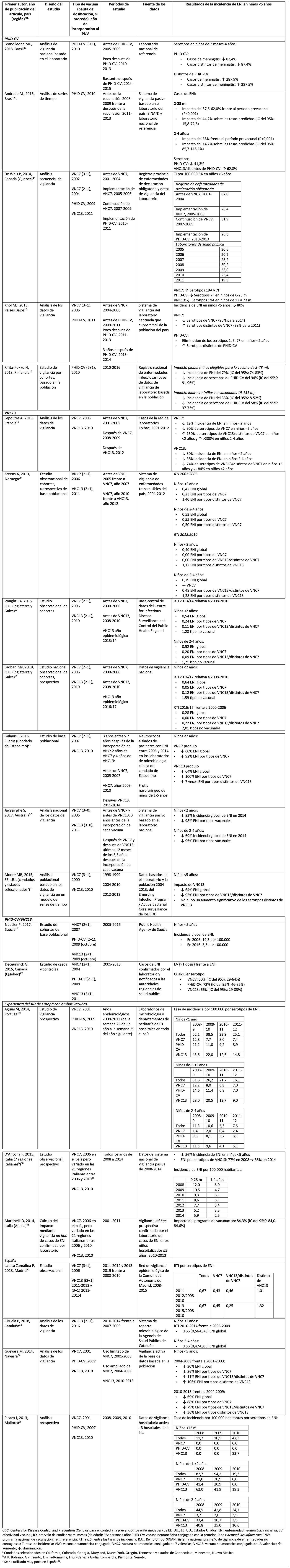
In Brazil, according to a time series study 3 years after it was introduced in 2010, PHiD-CV had an impact on IPD cases in children <4 years, particularly on the serotypes contained in the vaccine, while non-vaccine serotypes had increased.12 Similarly, a subsequent laboratory surveillance study reported that, 5 years after introducing PHiD-CV, the serotypes contained in the vaccine had decreased significantly, thus changing the profile of the remaining serotypes causing IPD.13 Another important finding was that, 3 years after the introduction of PHiD-CV, there was a substantial impact on the serotypes contained in the vaccine in the unvaccinated population (18–64 and =65 years), which denotes herd immunity.13 Serotypes 19A, 6C and 3, which had increased substantially in all age groups, were the main causes of the remaining IPD cases in Brazil.13
A Canadian study showed an impact on the overall incidence of IPD (mainly by serotypes 7F and 19A) in children <5 years exposed to PHiD-CV.14
In the Netherlands, where PCV7 was implemented in 2006 and replaced by PHiD-CV in 2011, there was an 80% reduction on the global incidence of IPD in children<5 years.15
A Finnish cohort study of children =6.5 years showed that, 6 years after the implementation of PHiD-CV in 2010, there was 79% reduction on the overall incidence of IPD, with a 94% reduction in the incidence of the serotypes included in the vaccine.16 There was 40% reduction on the incidence of IPD (from 6.3 to 3.8 per 100,000 people/year), mainly due to the 95% relative decrease in the incidence of serotype 6A; the impact on the reduction of the incidence of serotype 19A was not statistically significant.16
In 2018, a Belgian brief report indicated that, following the change from PCV13 to PHiD-CV, the incidence of serotype 19A-related IPD had increased by 10 among children =2 years.17 However, this study was based on typification data from a single year rather than several years of surveillance. In addition, the authors did not take into account the similar increase in IPD observed before the change nor did they consider the decrease in the incidence of IPD in children <1 year associated with PHiD-CV.18
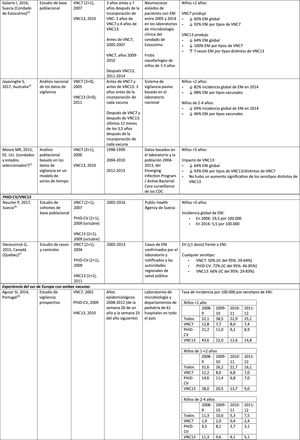
Impact of PCV13 in different populationsA study in France found that, 2 years after the introduction of PCV13, which replaced PCV7 in 2010, there was an impact on the incidence of all-type IPD and IPD due to non-PCV serotypes.19 However, the pre-vaccine period was short and did not allow for adequate assessment of pre-vaccine trends.19
A Norwegian retrospective population-based observational study observed an impact on the overall incidence of IPD, especially in children <2 years (60%, from 20 to 8.1 per 100,000), 2 years after the introduction of PCV13.20
U.S. researchers used a time series model to compare actual versus expected incidence if PCV13 had never replaced PCV7.21 They noted that PCV13 would have had an impact of a 64% reduction on overall incidence of IPD in children <5 years. A limitation of this study is that, according to the model, the incidence of IPD due to the PCV13 serotypes not contained in PCV7 would continue to increase after the introduction of PCV13.21
A study conducted in Australia compared equivalent periods of 3.5 years after the introduction of PCV7 and of PCV13.22 The authors described an 11% reduction in the overall incidence of IPD at all ages after the introduction of PCV13, compared with the pre-PCV13 period. When comparing equivalent PCV7 periods, the impact was 42%. After the introduction of PCV13, it was shown that only the impact on the incidence of IPD due to serotype 19A was of a similar magnitude to that observed after the introduction of PCV7.22
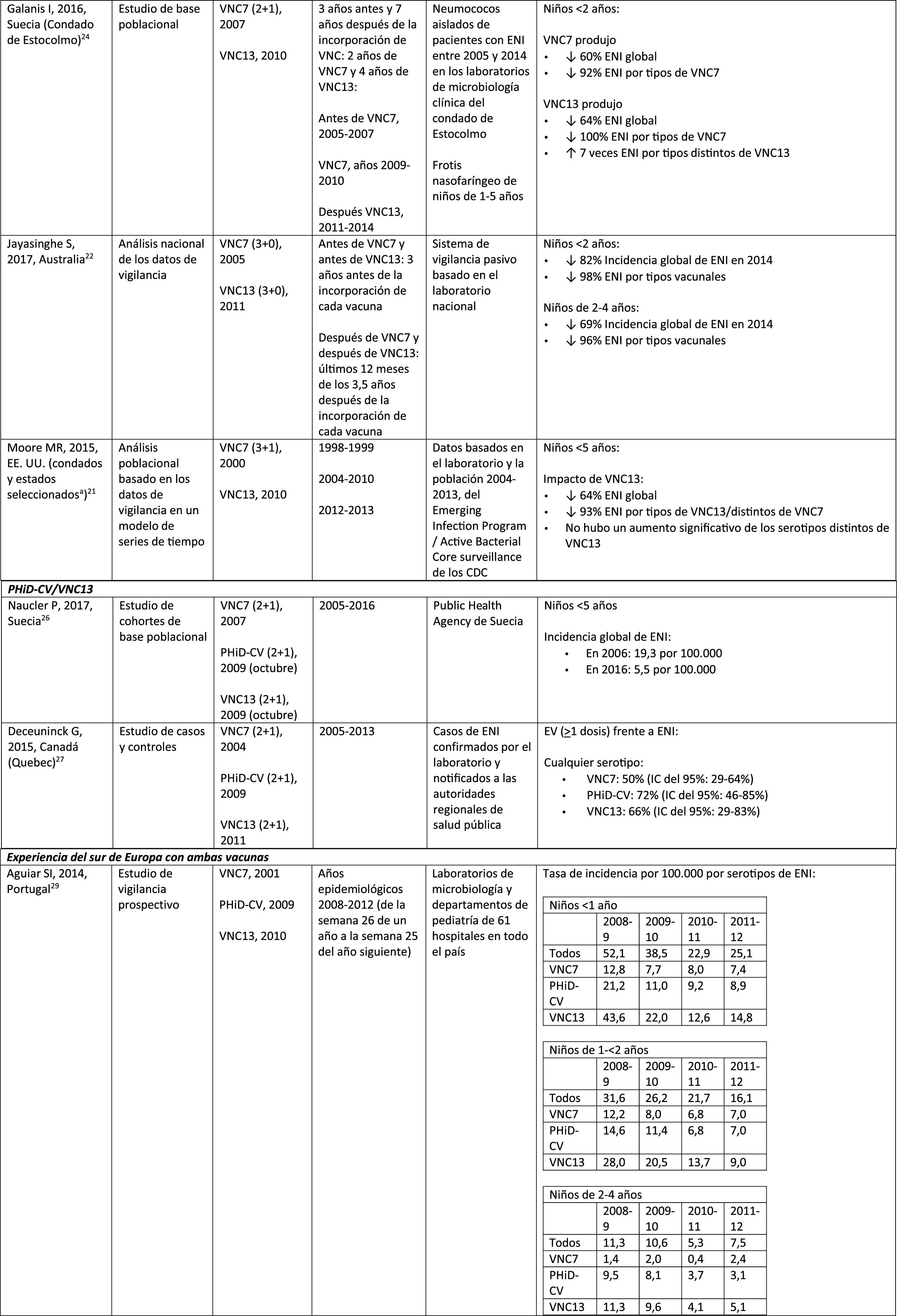
In 2 studies, one from England and Wales23 and the other from Sweden,24 the impact of PCV13 was assessed over the first 4 years from its introduction (2010). The England and Wales study reported a 32% reduction in overall IPD incidence in all age groups, compared to the pre- PCV13 period. There was a 69% reduction in the incidence of IPD due to the serotypes of PCV13 not contained in PCV7 and the greatest reduction occurred in children <5 years of age (91% in children aged 2–4 years and 89% in children <2 years).23 With respect to the additional PCV13-specific serotypes, the impact was a significant reduction in the incidence of serotypes 1, 6A, 7F and 19A in all age groups (in children <5 years of age, the reductions were 91%, 100%, 91% and 91%, respectively) but serotype 3 showed annual fluctuations and was only significantly reduced in the 5–64 (59%) and >65 age groups (44%).23 In the Swedish study, a statistically significant impact on the overall incidence of IPD was observed in children <2 years compared to the pre-PCV7 era and the impact was not statistically significant compared to the post-PCV7 period.24 In line with the England and Wales study, there was an impact on the incidence of IPD due to all PCV13 serotypes, except serotype 3, which increased. Among the serotypes that decreased, only 7F did so significantly post-PCV13, compared to post-PCV7.24
Seven years after the introduction of PCV13 in England and Wales, the incidence of IPD was only 7% lower (incident rate ratio [IRR] .93; 95% confidence interval [CI] .89–.97) than pre-PCV13 (10.13 per 100,000).25 The large reductions in IPD with PCV13 that were achieved 4 years after the introduction of the vaccine were affected by the rapid increase (IRR: 1.97; 95% CI: 1.86–2.09) in the incidence of IPD due to non-PCV13 serotypes (7.97 per 100,000).25 Serotypes 8, 12F and 9N represented 40% of total IPD.25
Comparison of the impact of PHiD-CV and PCV13 in the same populationA population-based cohort study in Sweden compared the incidence of IPD caused by specific serotypes before and after the introduction of PHiD-CV and PCV13 in 2009.26 Both vaccines were associated with an impact on serotype 6A (83% reduction). There was no positive impact on serotype 3 with either vaccine. Serotype 19A reached an incidence of 1.1 per 100,000 population in children <5 in the counties where PHiD-CV was used compared to no cases in those where PCV13 was used. The impact of the 2 vaccines on the overall incidence of IPD was not statistically different (RRI comparison: 1.00; 95% CI: .89–1.12).26
A Canadian study evaluated the effectiveness of the 3 PCV in laboratory-confirmed cases in children =5 by reviewing their vaccination records.27 Effectiveness on overall IPD was 72% with PHiD-CV, 66% with PCV13 and 50% with PCV7; effectiveness against serotype 19A was 71%, 74% and 42%, respectively.27
Experience with both vaccines in southern EuropeCurrent evidence from surveillance data from southern Europe suggests an impact on the incidence of IPD after the introduction of the conjugate vaccines.28
An active surveillance study in Portugal showed that the impact was a significant reduction of the overall incidence of IPD in children <5 on comparing the pre-PHiD-CV and post-PCV13 periods (2009 and 2010, respectively).29
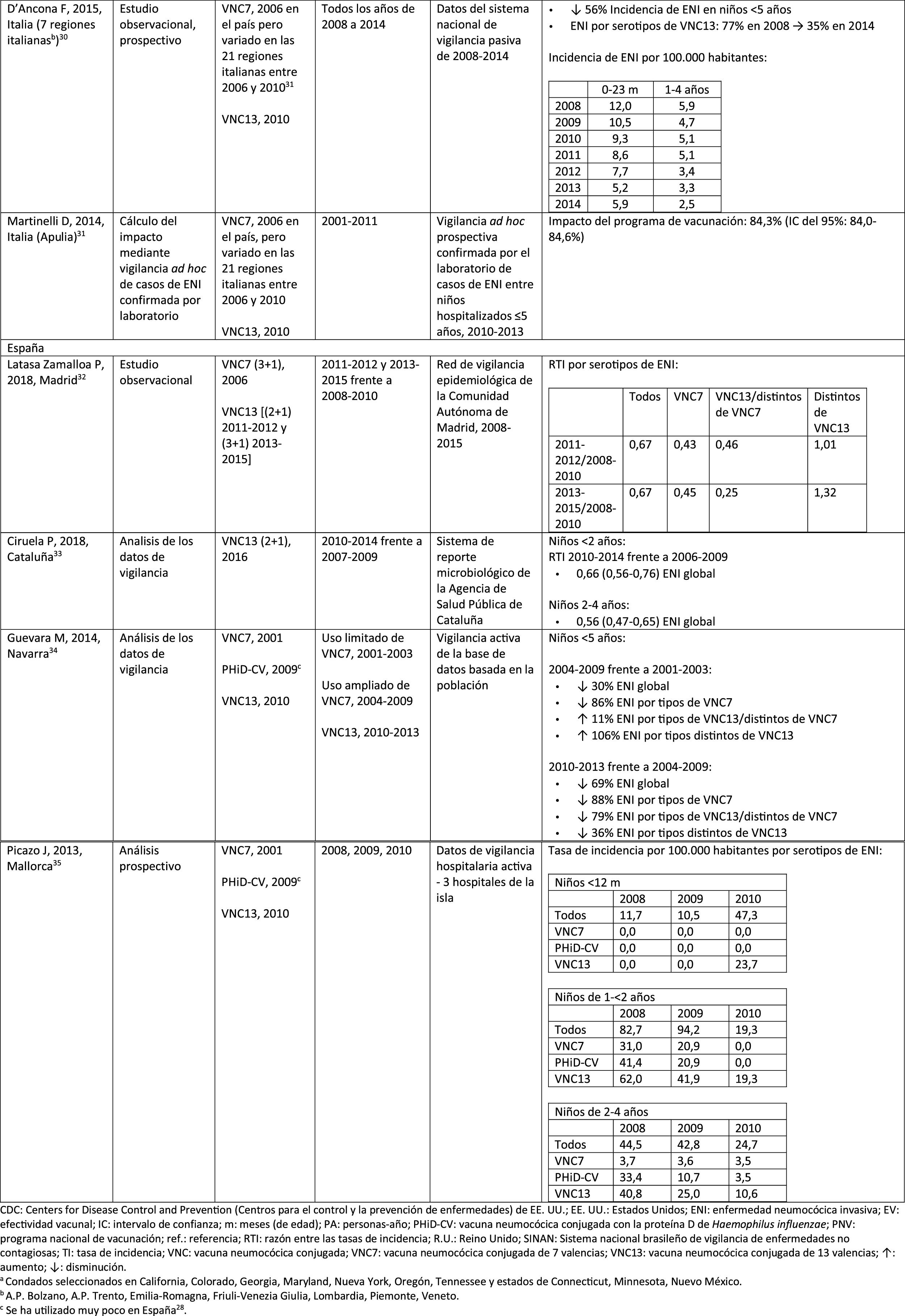
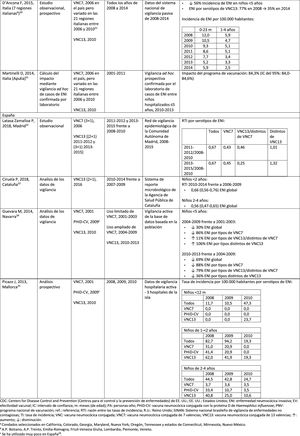
In Italy, evidence shows that the introduction of PCV13 had a significant impact in reducing the incidence of IPD in children <530 and that the general vaccination programme with PCV proved highly effective.31
In Spain, a study conducted in Madrid reported a reduction on the overall incidence of IPD after the introduction of PCV13.32 Serotype 3 was the most prevalent of the vaccine serotypes. However, these results are limited by the lack of data from periods pre-PCV7.32
In Catalonia, a recent study showed an impact of 26.2% reduction in overall IPD in the period when PCV13 was used with intermediate vaccination coverage in children <2 (64%). The greatest impact of reduction was observed in children from 2 to 4 years of age (44.5%).33
A surveillance study in Navarra with data based on the active population assessed the impact of PCV13 3 years after it was introduced to replace PCV7.34 The study showed an impact on the incidence of IPD in children <5. The impact was an 81% reduction on the incidence of IPD caused by the vaccine serotypes.
A prospective surveillance study in Mallorca examined the progress of IPD cases in children <15 years who required hospitalisation after PCV had been put on the private market.35 The study showed an impact of =50% due to the significant reduction in the incidence of IPD due caused by the vaccine serotypes included in PHiD-CV and VNC13.35 Although all paediatric hospital admissions due to IPD in the period 2008–2010 were included in the study, the conclusions are limited by the small number of patients included (66 cases of IPD of all ages, only 25 cases among children =2 years).35
PneumoniaImpact of PHiD-CV in different populationsA 2016 review of the impact of incorporating PHiD-CV into the Brazilian infant immunisation programme in 2010 found 8 reports showing the impact of PHiD-CV in reducing CAP by 40%, in reducing hospitalisation rates due to pneumonia by 12.7% and 28.7% and a reduction of 16.9% in children aged 2–23 months compared to mortality secondary to other respiratory causes.36
In 2017, a time series study of national surveillance data by hospital admission in Brazil after the introduction of PHiD-CV showed significant decreases in hospitalisation rates due to pneumonia in all age groups up to the age of 49, ranging from 13.9% and 22.2%. The impact of PHiD-CV on the reduction in hospitalisation of children <5 ranged from 17.4% and 26.5%.37
The impact of PHiD-CV on the reduction of cases of pneumonia was also detected in 2 Finnish studies, the first population-based national impact studies to document the direct and indirect effects of routine PHiD-CV vaccination among vaccine-eligible and unvaccinated children.38,39 Since the introduction of PhiD-CV in 2010, these studies revealed a substantial impact on the reduction of the total cases of pneumonia and pneumonia treated in hospital among vaccine-eligible children and older unvaccinated children.39
Impact of PCV13 in different populationsA study in England, using data from all hospital admissions, compared the incidence of pneumococcal disease-specific variables over a 24-month period pre-PCV7 versus the post-PCV13 period (2013–2015).40 Relative reductions in pneumococcal pneumonia were observed in all age groups, regardless of the presence of risk factors. In the case of pneumonia of unspecified cause, decreases were recorded among children <15 years and the maximum impact of 34% reduction was in children <2 years.40
A Scottish study estimated an impact of 30% reduction on all-cause pneumonia hospitalisation rates in children <2 years after the introduction of PCV13 in 2010, compared to pre-PCV7 (2006). However, the corresponding rates increased among adults >75 years. Deaths due to pneumonia and hospitalisation due to pneumococcal pneumonia decreased in all age groups.41
Comparison of the impact of PHiD-CV and PCV13 in the same populationA systematic review of studies from Latin American and Caribbean countries examined the impact of vaccination on children <5 years.42 However, none of the studies included compared the impact of PHiD-CV and PCV13, and there was a high degree of heterogeneity that prevented a meta-analysis.42 The review showed an impact of between 8.8% and 37.8% reduction for radiographically confirmed pneumonia, and between 7.4% and 20.6% reduction for clinical pneumonia. Results varied with age, case definition and type of vaccine.42 Overall, this systematic review did not show that one vaccine was superior to another with regard to hospitalisations for IPD, pneumonia and meningitis in children <5 years.42
A systematic review and meta-analysis of studies published between 2000 and 2016 showed that both PHiD-CV and PCV13 had a significant impact in reducing hospitalisation rates for clinical pneumonia in children <24 months by 16% (IRR: .84, 95% CI: .78–.90) with PHiD-CV and 29% (IRR: .71, 95% CI: .70–.72) with PCV13.43
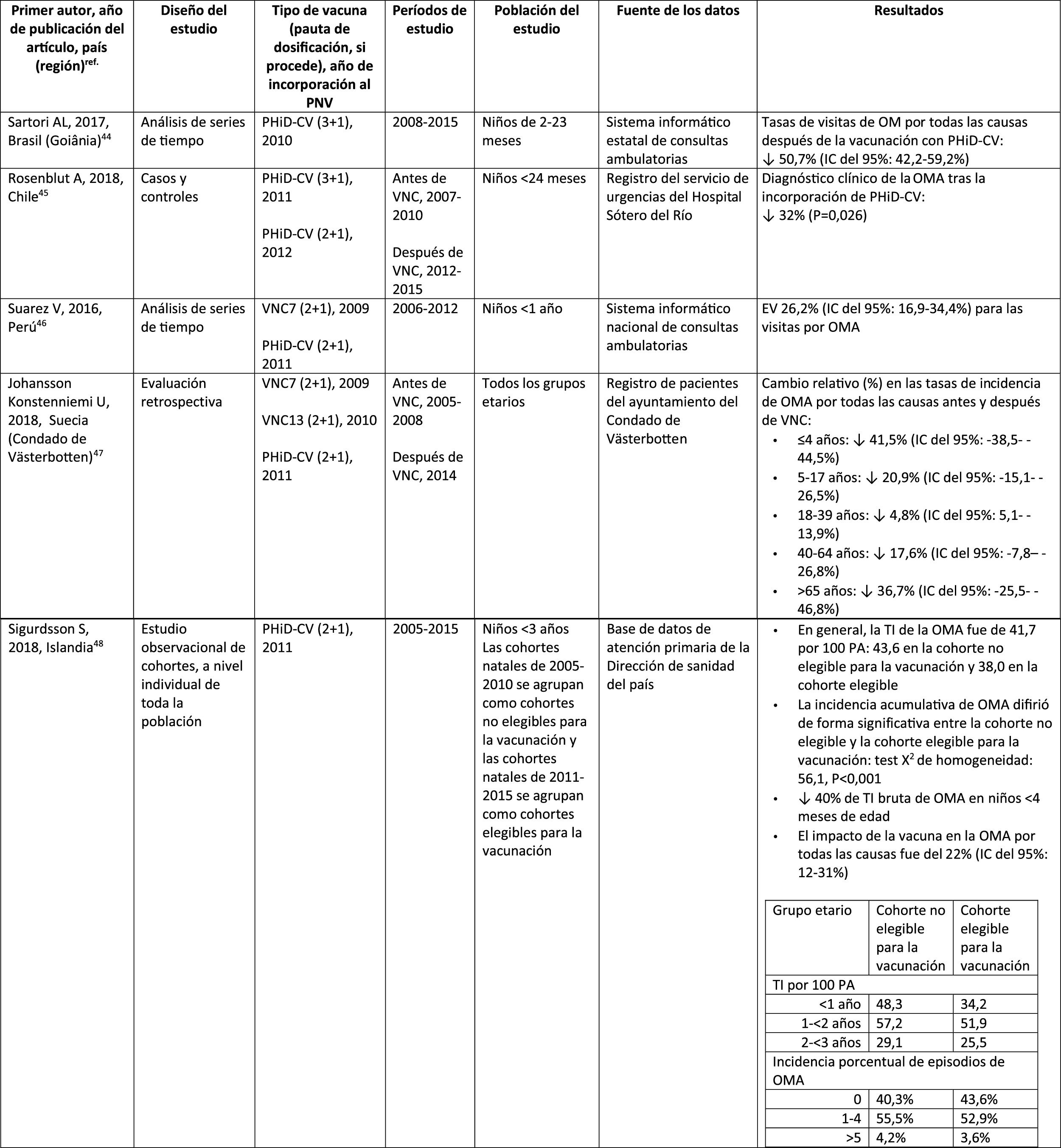
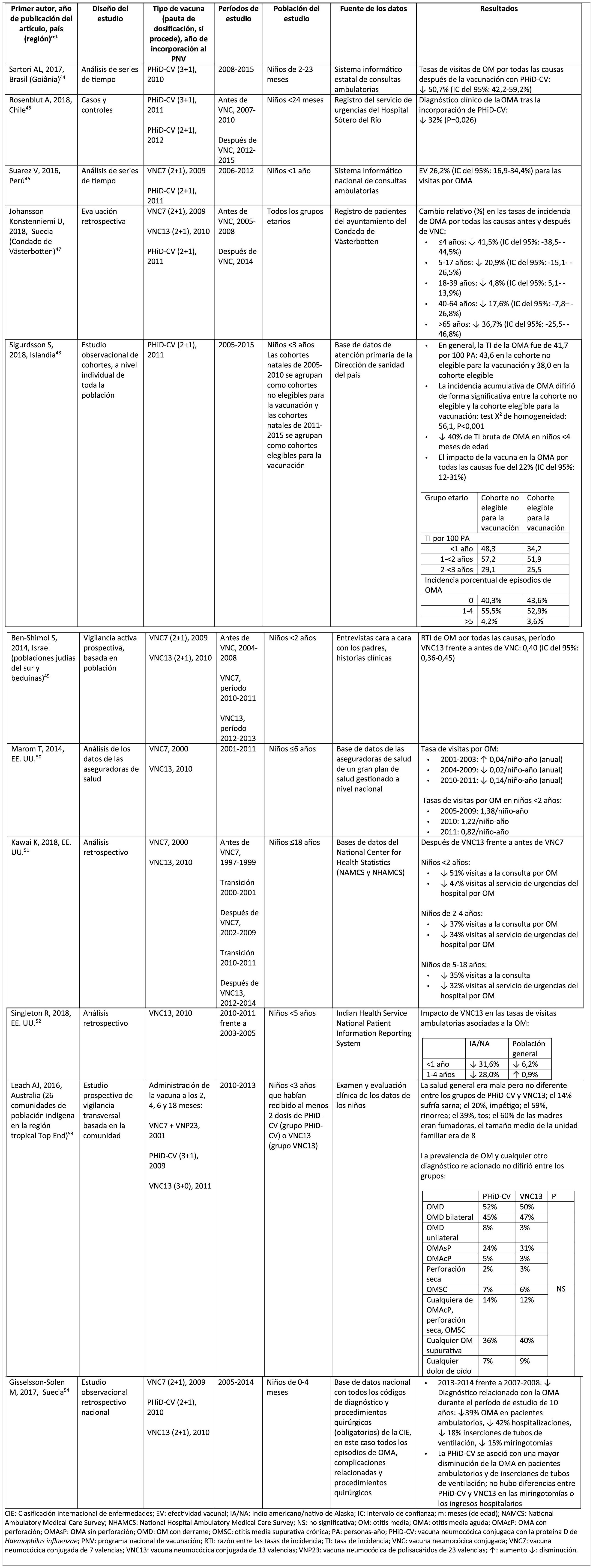
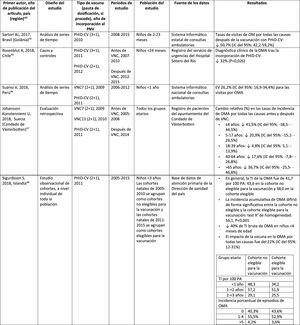
Acute otitis mediaImpact of PHiD-CV in different populationsIn a time series study, conducted in the municipality of Goiânia in Brazil, the impact of PhiD-CV on all-cause OM was a reduction of 43.0% (95% CI 41.4–44.5%) among children aged from 2 to 23 months.44
In a study from Chile, which evaluated the frequency of AOM in children <24 months attended in a hospital emergency department, a 32% effectiveness (p=.026) for AOM was found after the introduction of PHiD-CV into the Chilean national vaccination programme.45
In Peru, the rates of outpatient visits for AOM among children <1 year decreased significantly, corresponding to a combined impact (CNV7 and PHiD-CV) of a reduction of 26.2% (95% CI 16.9–34.4%).46
In Sweden (county of Västerbotten), where PCV7 was replaced by PCV13 in 2010 and by PHiD-CV in 2011, the impact was a significant reduction on the incidence of all-cause AOM in children =4 years (41.5%, 95% CI: -38.5–44.5%) after introducing the PCVs.47
A study in Iceland compared the incidence of all-cause AOM in children <3 years pre- and post-PHiD-CV (2011) and showed that PHiD-CV had a significant impact in reducing all-cause AOM.48 PHiD-CV significantly decreased the frequency of the first 2 episodes and the incidence of AOM in all age groups. The greatest decrease in incidence was in children <4 months (40%, 95% CI 31–49%), an age group that is too young for vaccination, suggesting herd immunity.48
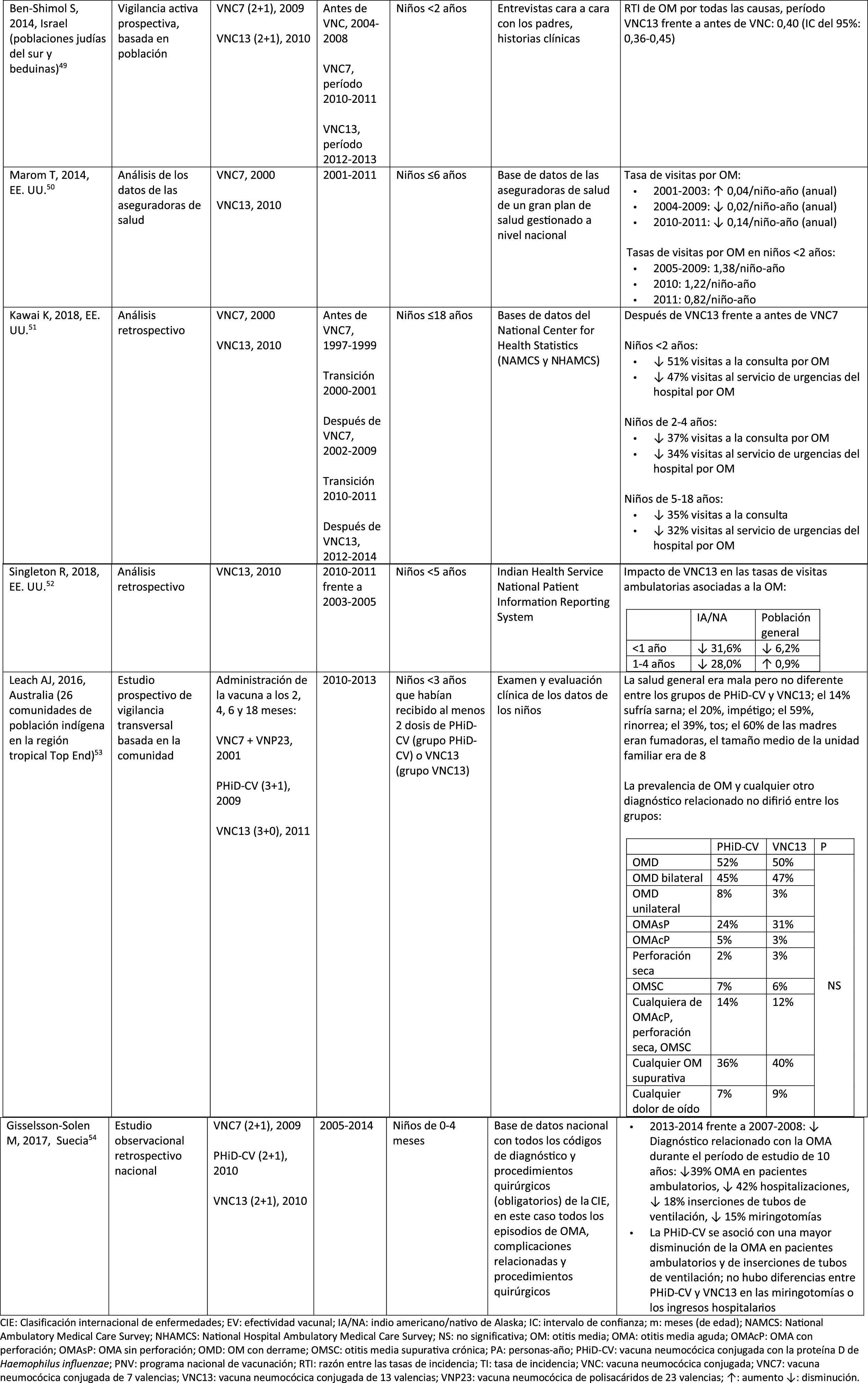
Impact of the PCV13 in different populationsIn 2014, 2 studies, one from Israel49 and the other from the USA.,50 showed that PCV13 had a substantial impact OM. The Israeli study reported a 60% reduction in the incidence of all-cause OM post- PCV13 (2010), compared to the period pre-PCV7 (2009).49 The US study found an impact on paediatric consultations due to MO, particularly among children <2 years, after the introduction of PCV13 (2010).50
In 2018, 2 further US studies were published, one on outpatient visits associated with OM51 and the other on cases of OM in American Indian and Alaska Native (AI/AN) children.52 In the study on annual outpatient visits due to OM there was a significant impact on medical visits (35%–51% reduction), as well as visits to the emergency department (32%–47% reduction), particularly in children <2 years, during the pre-PCV13 period (2012–2014), compared to the pre-PCV7 period (1997–1999).51 The study on AI/AN children indicated that outpatient visits for OM decreased after the introduction of PCV13. Among AI/NA infants, the rate of outpatient visits for OM after the introduction of PCV13 (2010–2011) was 130.5 per 100 infants/year, 1.6 times higher than the visit rates among other US infants.52
Comparison of the impact of PHiD-CV and PCV13 in the same populationSurveillance data from a population of indigenous Australian children <3 years with a high prevalence of OM showed that general health and hearing health were statistically similar, irrespective of the administration of PHiD-CV or PCV13.53 The prevalence of suppurative OM was statistically similar among the PHiD-CV and PCV13 cohorts in all of the <12 month, 1–<2 year and 2–<3 year age groups.53
In a Swedish observational study, which evaluated AOM-related diagnoses in children aged from 0 to 4 over a period of 10 years that covered the pre- and post- PCV era, it was observed that in 2014 the risk ratio of outpatient episodes of AOM and intubations had reduced by 25% and 22% respectively, in the counties where PhiD-CV was used compared to those where PCV13 was used (p<.001).54
Cross-protectionCross-protection of PhiD-CV against serotype 19A in vaccinated childrenA study of pneumococcal strains isolated from Brazilian children during the first 2 years after marketing demonstrated cross-protection with PhiD-CV and its effectiveness against serotype 19A.55
A study in the Netherlands on surveillance data covering the periods pre-PCV7 (2004–2006) and post-PhiD-CV (2011–2014) did not reach a conclusion on cross-protection against serotype 19A.15 Although there was an impact with PHiD-CV on the incidence of serotype 19A-related IPD compared to the PCV7 cohort, this was no different to that recorded for PDI caused by serotypes unrelated to the vaccine.15
In the same year (2015) a Canadian study was published, in which PHiD-CV replaced PCV7 in 2009 and PCV13 replaced PHiD-CV in 2011; the study reported the cross-protection of PhiD-CV against IPD caused by serotype 19A, after an analysis of data obtained over a period of 8 years from the PCV7 era to the post-PCV13 era.27
In addition, a Finnish study suggested that 3 years after the introduction of PHiD-CV there was cross-protection against serotype 19A IPD in vaccine-eligible children.56 However, this study's long-term follow-up (6 years after the introduction of PHiD-CV) showed no significant impact of PhiD-CV on IPD caused by serotype 19A.16 The age of the children was associated with the calendar period, as 19A IPD cases were mainly recorded in older children and occurred at the end of the follow-up period. This could be due to increased pressure from 19A infection and/or a possible initial cross-protective effect caused by the 19F component of PhiD-CV, which gradually decreased with age, which would explain why the vaccinated children were susceptible to serotype 19A IPD. However, the study drew no conclusions given the limited number of cases and the secular trend of the 19A cases.16
Cross-protection of PHiD-CV against serotype 6ASome studies point to possible cross-protection of PHiD-CV against 6A. However, the current evidence is heterogenous. Early post-marketing data did not show any cross-protection of PHiD-CV against serotype 6A.55 However, the study sample contained only 24 cases of serotype 6A IPD and therefore these results should be interpreted with caution.55
Finnish data from the 3 years following the introduction of PHiD-CV indicated cross-protection, with impact on the incidence of serotype 6A IPD in the cohort of vaccination-eligible children.56 Long-term data confirmed cross-protection against serotype 6A, with a significant impact (95% reduction) on the incidence of serotype 6A IPD.16 However, it is not possible to conclude indirect protection against 6A, as the number of cases in older ineligible children was too small to support such a claim.16
Cross-protection of PCV13 against 6CCross-protection of PCV13 against 6C has been proposed due to serotype 6A included in the vaccine, however in general the current evidence is contradictory. Analysis of serum samples from children one month after their first vaccination with either PCV7 or PCV13 showed a significantly different response to serotype 6C between the PCV7 and PCV13 cohorts, suggesting that most functional cross-protective response to 6C was mediated by PCV13 serotype 6A, this assumption is also supported by the greater correlation found between serotypes 6A and 6C than between serotypes 6B and 6C (Pearson correlation r: .78 and .21, respectively).57
A Norwegian study evaluated the changes in the incidence of IPD after PCV7 was replaced by PCV13 and a decrease in the incidence of serotype 6C-related IPD was detected, which suggests cross-protection.20 By contrast, another study that also compared the incidence of IPD by serotype between the PCV7 and PCV13 periods, found no significant changes in the incidence of serotype 6C-related IPD.23
Herd immunity and serotype replacementLong-term studies on the impact of PCVs on IPD, pneumonia and AOM have shown herd immunity but also an increase in non-PCV serotypes.16,24,25,40,47,48,58,59 An analysis of all hospital admissions in England pre- and post-PCV showed a significant reduction in cases of pneumococcal pneumonia in high-risk subjects.40 However, pneumonia due to unspecified microorganisms increased in high-risk subjects aged >65 (gross ratio between cases: 2.96), which shows susceptibility to the serotypes that replaced those of PCV13.40
Similarly, in an analysis of surveillance data obtained over 17 years in England and Wales, vaccination with PCV7 and PCV13 was found to provide herd immunity to IPD. However, IPD due non-PCV13 serotypes doubled after the introduction of PCV7 (from 3.85 to 7.97 per 100,000).25
A Swedish study showed the indirect protective effect for adults and children.24 However, no herd immunity was shown for older adults; 68% of IPD cases in this population were caused by non-PCV13 serotypes.24
Before the introduction of PHiD-CV in Finland, hospitalisations for all-cause pneumonia increased among adults with an annual rate of 2.4%.6 After the introduction of PHiD-CV, all-cause pneumonia started to reduce with an annual rate of 4.7% and a significant decrease of 6.7% was recorded among subjects =65 years, which suggests herd immunity thanks to the infant vaccination programme.60
In the Brazilian time series study, PHiD-CV vaccination was associated, 5 years after its introduction, with herd immunity among ineligible cohorts aged 10–49.37
DiscussionThe current evidence on both PHiD-CV and PCV13 shows the global impact of these vaccines against IPD, pneumonia and OM when included in national immunisation programmes in different geographical settings.61 This impact is the result of the reduction of disease caused by the serotypes included in each formulation plus the related serotypes. A variable increase in disease due to non-vaccine types can be seen and varies according to the geographical context. However, the net effect on the eligible vaccinated cohort has been demonstrated.61
Comparative data on the impact of PhiD-CV and PCV13 on all pneumococcal disease are currently lacking. Despite the abovementioned studies, it appears difficult to establish the differences between these 2 vaccines for a number of reasons. Surveillance systems not only differ between countries, but also between regions within the same country.12,14,20,21,34 Although the WHO recommends that the surveillance data set should include a period starting at least 2 years pre- and ending 5 years post-PCV,1 many studies only provide very short-term information that does not allow clear and robust conclusions to be drawn on the global impact of the vaccines.62 Consideration should be given to the variability that other confounding factors may introduce such as increased case reporting due to greater clinical suspicion of the disease following introduction of the vaccines and the changes in case definition over time on the incidence of disease.16,20,22,34 Other factors that make comparison of these studies difficult are the differences in the epidemiology of the disease and in diagnostic methods (use of polymerase chain reaction instead of culture) that vary according to geographical area or time period.12,20,22,34 Some aspects that also limit comparisons are the differences in vaccine coverage, heterogeneity in study design, difference in the prevalence of co-morbidity and other risk factors according to the country,22,24,28 and the fact that the review was not systematic.
For the abovementioned reasons, the most reliable comparisons are obtained through systematic reviews42,43 and studies, such as the sequential studies conducted in Quebec to compare the effectiveness of PCV7, PhiD-CV and PCV13 administered one after the other,27 or the comparative study of the impact of PHiD-CV and PCV13 on IPD in the Swedish population.26 Thanks to their stable nationwide surveillance system and the possibility for all 21 counties to choose the vaccine they wish to use separately, Sweden26 has been able to conduct a real-world assessment of the impact of the simultaneous use of PHiD-CV and PCV13, the results of which support the Quebec study27 in the sense that there is no difference in the impact of either PCV on the overall incidence of IPD and associated hospitalisation.
Reviews of the existing evidence by WHO and the Pan American Health Organization (PAHO) found no evidence of the superiority of one vaccine over the other in children <5 and both agencies recommended inclusion of PCVs in childhood vaccination programmes worldwide.63,64
ConclusionsPCVs have shown an impact on IPD, pneumonia and OM in different geographical settings. Given the heterogeneity in vaccination implementation strategies, vaccine coverage, forms of case reporting, it is difficult to assess the magnitude of the impact on the burden of pneumococcal disease for each vaccine. Comparative data have suggested that one vaccine does not outweigh the other in terms of impact on overall IPD. This concept has been adopted by independent international bodies such as the WHO and PAHO. It is, therefore, the impact on global pneumococcal disease that really matters beyond the serotypes contained in the formulations.
Fig. 1 summarises the context, results and impact of this literature review for health professionals.
DeclarationTrademarksSynflorix is a trademark of the GSK group of companies.
Prevenar 13 is a trademark of Wyeth LLC.
FundingGlaxoSmithKline Biologicals SA sponsored the literature review and the development and publication of the manuscript.
AuthorshipAll the authors participated in the design or the implementation of the literature review or in the analysis and interpretation of this review, and in preparing this manuscript. All the authors agreed to take responsibility for all aspects of the work so that any questions regarding the accuracy or completeness of any part of the article will be investigated and resolved appropriately. The work was carried out in accordance with ICMJE recommendations for the conduct, reporting, editing, and publication of scholarly work in medical Journals. The corresponding author assumed responsibility for submitting the final manuscript for publication.
Conflict of interestsXMPP has participated as an expert on the Advisory Committee in the GSK group of companies and has given lectures for this group. JNG, AE and JLGR are employees of the GSK group of companies. JNG and AE hold shares in the GSK group of companies. BT was an employee of the GSK group of companies at the time of the literature review and preparation of this manuscript. JCDM and XSL have no conflict of interest to report.
On behalf of GSK, the authors would like to thank the Business & Decision Life Sciences platform for editorial assistance and coordination of the manuscript. Grégory Leroux coordinated the development of the manuscript and editorial support. The authors also thank Athanasia Benekou (Business & Decision Life Sciences, on behalf of GSK) for her help in writing the manuscript.
Please cite this article as: De Moraes C, Pérez Porcuna XM, Nieto-Guevara J, Eisman A, Torres B, Gonzalez Redondo JL, et al. Vacunación frente a la enfermedad neumocócica con vacunas conjugadas: ¿qué es lo que verdaderamente importa? Vacunas. 2020;21:23–43.