This research intended to evaluate the antibody response to vaccination against hepatitis B during the first year of life. We also aimed to assess the association between the antibody response to hepatitis B vaccination and the polymorphism in HLA-G 14bp.
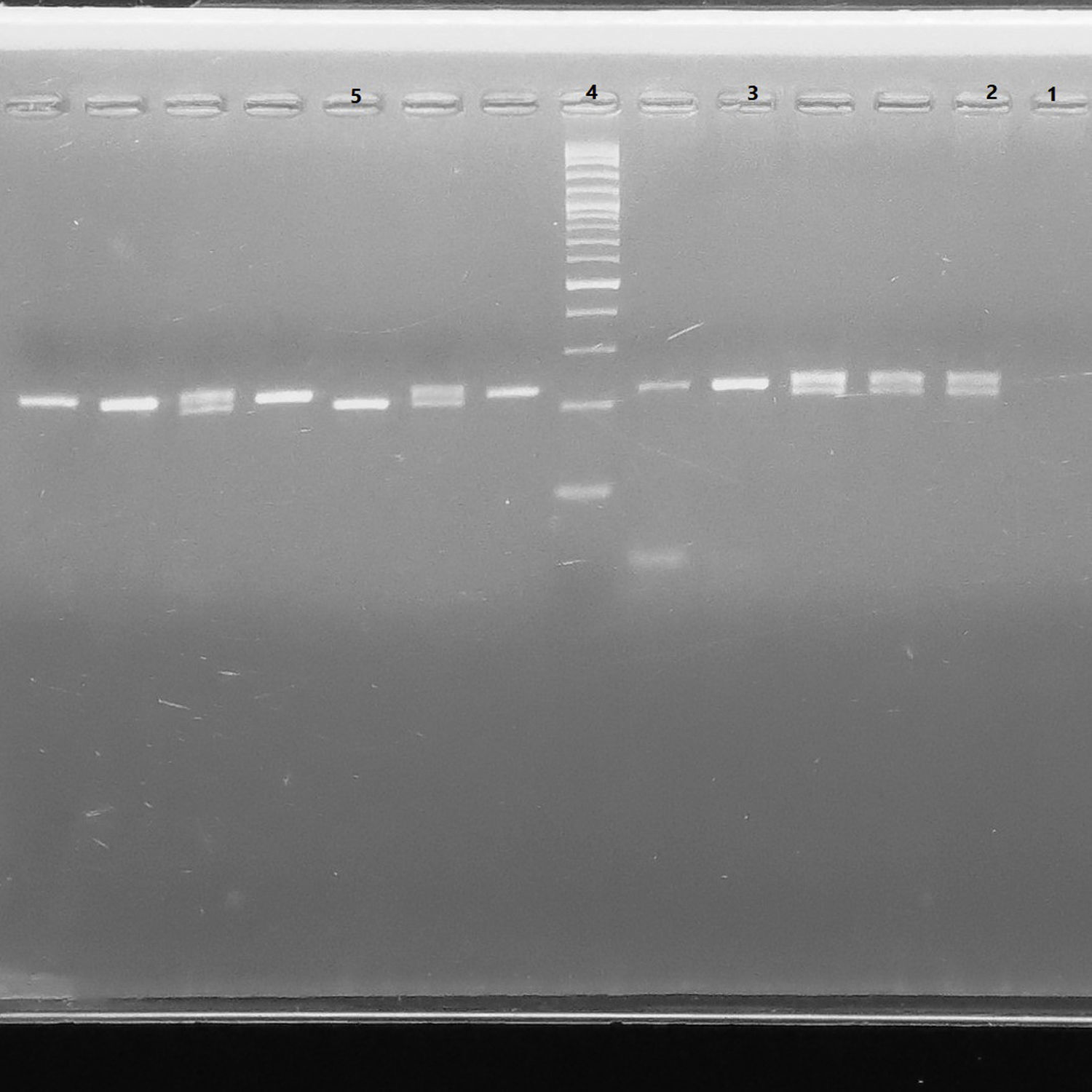
Material and methodsIn this comparative cross-sectional study, 93 infants that received the hepatitis B vaccine at 2, 4, and 6 months old according to the World Health Organization immunization schedules, were evaluated for their antibodies to HBsAg (anti-HBs) following vaccination. Their genomic DNA was extracted and examined for the polymorphism in HLA-G 14bp by PCR.
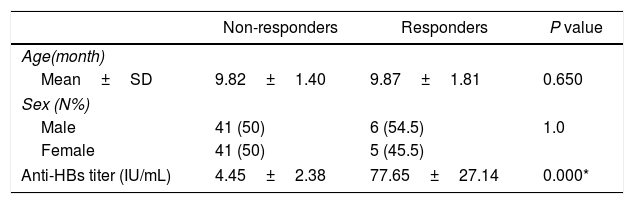
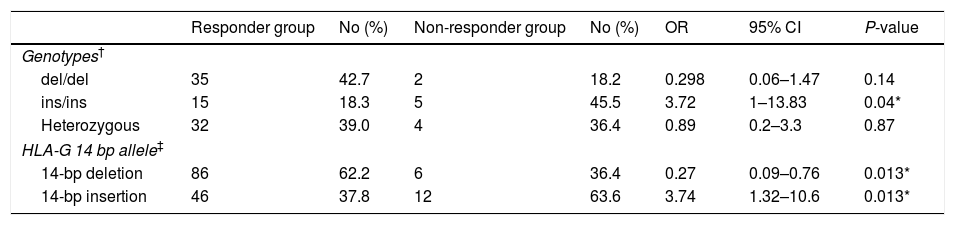
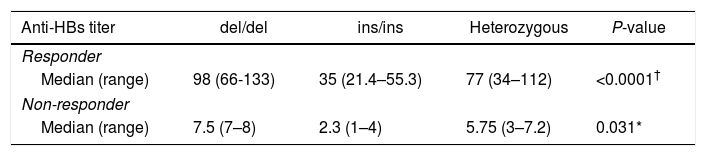
ResultsAmong 93 vaccinated infants, 11 infants (11.8%) were non-responders to vaccination against hepatitis B (anti-HBs<10IU/mL). The mean antibody titers for responders and non-responders were 77.65±27.14IU/mL and 4.45±2.38IU/mL, respectively. The 14-bp insertion allele was associated with an increased risk of failure o respond to hepatitis B vaccination (OR 3.72, 95% CI 1.32–10.6).
ConclusionThis study reported, for the first time, that ins/ins genotype may be a risk factor for non-responsiveness to hepatitis B vaccination.
El objetivo de este estudio fue evaluar la respuesta de anticuerpos a la vacuna frente a la hepatitis B durante el primer año de vida. También tratamos de evaluar la asociación entre la respuesta de anticuerpos a la vacuna frente a la hepatitis B y el polimorfismo en HLA-G 14bp.
Material y métodosEn este estudio transversal comparativo se evaluó el HBsAg (anti-HBS) de 93 niños que recibieron la vacuna contra la hepatitis B a los 2, 4 y 6 meses de edad, conforme a los programas de la Organización Mundial de la Salud, fueron evaluados por sus anticuerpos frente a HBsAg (anti-HBs) después de la vacunación. Se extrajo su ADN genómico. examinándose el polimorfismo en HLA-G 14bp mediante la prueba PCR.
ResultadosEntre los 93 niños vacunados, 11 niños (11,8%) no respondieron a la vacuna frente a la hepatitis B (anti-HBs<10UI/mL). Los títulos de anticuerpos medios para respondedores y no respondedores fueron de 77,65±27,14 y 4,45±2,38UI/mL, respectivamente. El alelo 14bp inserción se asoció a un incremento del riesgo de fallo de respuesta a la vacuna frente a la hepatitis B (OR: 3,72; IC 95%: 1,32-10,6).
ConclusiónEste estudio reportó, por vez primera, que el genotipo ins/ins puede constituir un factor de riesgo para la no respuesta a la vacuna frente a la hepatitis B.