In COVID 19, an aggressive inflammatory response called cytokine release storm has been described. It is mainly mediated by the activation of Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), mainly observed in critically ill patients. Among the multiple treatments proposed throughout these two years of pandemic, we highlight the use of Tocilizumab (TCZ).
ObjectivesTo evaluate in-hospital mortality, transfer to critical care unit (CCU), invasive mechanical ventilation requirement (IMV), and hospital stay in patients treated with TCZ versus conventional treatments (CT).
MethodsRetrospective, descriptive, and analytical cohort study. Hospitalized patients, May to July 2021. Branches: treated with TCZ versus CT. Statistical analysis: Epi Info 7.2.
ResultsNinety patients, 51 TCZ branch and 39 CT branch. Age 48.2 years (± 11.7), males 74 (82.2%). Comorbidities 66 (73.3%): High blood pressure (HBP) 32 (35.6%), Diabetes mellitus 13 (14.4%), BMI>30, 51 (56.7%). Medical Clinic Admission (MC) 85 (94.4%). Days post-symptom onset 7.9 (± 2.6). Severity of COVID 19: severe 61 (67.8%), critical 26 (28.9%). CCU admission 26 (29.9%). IMV 16 (17.8%). Deaths 7 (7.9%). Hospital stay 12.9 (± 6.6) days. Comparative analysis TCZ versus CT: MC admission 50 (98%) versus 35 (89.7%) p .08. CCU admission 12 (23.5%) versus 14 (38.9%) p .1. IMV 4 (7.8%) versus 12 (30.8%) p .005. Death 1 (2.0%) versus 6 (15.8%) p .02. Mortality in univariate analysis (p<.05): APACHE II, BMI>30, TCZ, IMV, and CCU admission. TCZ was a protective factor against RR of death .86 (.74–.99). The IMV requirement was a RR factor for death 2.0 (1.23–3.42). Cox logistic regression, independent survival factors: use of TCZ, absence of obesity, and no IMV, p .0000.
ConclusionsIMV was found to be a risk factor for mortality. TCZ did not show a decrease in CCU requirement, but it did prove to be a protective factor against mortality. However, this is a non-randomized study so it should be interpreted with caution.
En COVID-19 se ha descrito una respuesta inflamatoria agresiva denominada tormenta de liberación de citocinas. Está mediado principalmente por la activación de la interleucina-6 (IL-6) y el factor de necrosis tumoral alfa (TNF-α), observado principalmente en pacientes críticos. Entre los múltiples tratamientos propuestos a lo largo de estos 2 años de pandemia, destacamos el uso de tocilizumab (TCZ).
ObjetivosEvaluar la mortalidad hospitalaria, el pase a una unidad de cuidados críticos (UCI), la necesidad de ventilación mecánica invasiva (VMI) y la estancia hospitalaria entre pacientes tratados con TCZ vs. tratamientos convencionales (TC).
Material y métodosEstudio de cohorte retrospectivo, descriptivo y analítico. Pacientes internados de mayo a julio de 2021. Ramas: tratados TCZ vs. TC. Análisis estadístico: Epi Info® 7.2.
ResultadosNoventa pacientes, 51 en la rama TCZ y 39 en la rama TC. Edad 48,2 años (±11,7), masculinos 74 (82,2%). Comorbilidades 66 (73,3%): HTA 32 (35,6%), diabetes mellitus 13 (14,4%), IMC>30 51 (56,7%). Ingreso en clínica médica (CM) 85 (94,4%). Días desde el inicio de los síntomas 7,9 (±2,6). Grado COVID-19: severo 61 (67,8%), críticos 26 (28,9%). Pase a la UCI 26 (29,9%). VMI 16 (17,8%). Óbitos 7 (7,9%). Estancia hospitalaria 12,9 (±6,6) días. Análisis comparativo TCZ vs. TC: Ingreso en CM 50 (98%) vs. 35 (89,7%) p 0,08. Pase a la UCI 12 (23,5%) vs. 14 (38,9%) p 0,1. VMI 4 (7,8%) vs. 12 (30,8%) p 0,005. Óbito 1 (2,0%) vs. 6 (15,8%) p 0,02. Análisis univariado mortalidad (p<0,05): APACHE II, IMC>30, TCZ, VMI y pase a la UCI. TCZ resultó factor protector ante óbito RR 0,86 (0,74-0,99). La necesidad de VMI resultó factor de riesgo de morir RR 2,0 (1,23-3,42). Regresión logística de Cox factores independientes sobrevida: uso TCZ, ausencia obesidad y no VMI, p 0,0000.
ConclusionesLa VMI resultó ser un factor de riesgo de mortalidad. TCZ no demostró disminuir requerimiento de la UCI, pero sí ser factor protector ante mortalidad. No obstante, es un estudio no aleatorizado por lo que debe interpretarse con precaución.
In December 2019, a severe acute respiratory syndrome associated with pneumonia caused by a new coronavirus called SARS-COV2 was identified in Wuhan (China). It spread rapidly and has become a public health challenge.1 Until August 2021, around 214 million people have been diagnosed with this virus, which has caused more than 4.4 million deaths worldwide. Around 25% of patients suffering from COVID 19 (Corona Virus Disease 2019) experience serious complications which lead to the requirement of intensive care unit admission, multiple organ failure and even death.1–4
In this disease, an aggressive inflammatory response called cytokine release storm has been described. It is mainly mediated by the activation of Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), mainly observed in critically ill patients. Among the multiple treatments proposed throughout these two years of pandemic, we highlight the use of tocilizumab (TCZ).1–9
TCZ is a monoclonal antibody subclass IgG1 that inhibits IL-6 soluble receptor, and therefore prevents the activation of the previously mentioned inflammatory response. This drug is widely approved for the treatment of different rheumatic diseases such as rheumatoid arthritis and juvenile idiopathic arthritis, among others.1–3,6,7,10
The National Institutes of Health (NIH), Chinese Health Commission and the Italian Medicines Agency (AIFA), among others, include this drug as a treatment for severe COVID 19.1,11–13
As it is a high-cost drug in the study phase, its availability and use depends on the criteria of each health care provider. The objective of this study is to evaluate in-hospital mortality, transfer to critical care unit (CCU), invasive mechanical ventilation requirement (IMV) and hospital stay between patients treated with Tocilizumab (TCZ) versus conventional treatments (CT).
MethodsStudy designA retrospective, observational, analytical cohort study was carried out, using data captured in electronic health records. We included all those patients admitted to Internal Medicine and critical care units services of our institution (level 3), located in the province of Mendoza (Argentina), between May 1 and July 31 of the year 2021.
After the Institution implemented the use of Tocilizumab as a compassionate treatment for COVID 19, the Teaching and Research Committee of our hospital approved the conduct of this study.
ParticipantsInclusion and exclusion criteria were developed:
Inclusion criteria: over 18 years of age, confirmation of COVID 19 through RT PCR or Ag SARS-COV2, and the signed informed consent by the patient or patient's relatives. Associated with a disease course of less than 15 days, presence of severe or critical pneumonia (according to WHO criteria, Table 1), and an increase in at least one inflammation marker (CRP>75mg/l, ferritin>1000ng/ml, IL-6>14pg/ml, LDH>300IU/l).
WHO definitions of disease severity for COVID-19.
| Disease severity | Criteria |
|---|---|
| Critical | Acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other condition that would require the provision of life-sustaining therapies (such as mechanical ventilation) or vasopressor therapy |
| Severe | Oxygen saturation<90% on room airSigns of pneumoniaSigns of severe respiratory distress (accessory muscle use, inability to complete full sentences, respiratory rate>30 breaths per minute) |
| Non severe | Absence of any criteria for severe or critical COVID-19 |
Therapeutics and COVID-19: living guideline, 14 July 2022. Geneva: World Health Organization; 2022 (WHO/2019-nCoV/therapeutics/2022.4). Licence: CC BY-NC-SA 3.0 IGO.
Exclusion criteria: pregnancy, lactation, signs and/or symptoms of associated bacterial/fungal superinfection, previous viral infections such as HIV, Hepatitis B, Hepatitis C; use of previous treatment with immunosuppressants, immunocompromised state due to any cause, increase in transaminases greater than 5 times the normal range (before TCZ infusion), risk of intestinal perforation, stage 4 or 5 chronic renal failure, hemoglobin less than 8mg/dl, white blood cell count less than 2000cells/mm3, platelet deficiency less than 50,000/mm3, and the patient or patient's relative refuse to sign the informed consent.
ProceduresAll patients meeting the previous criteria were included in the study. Two branches were defined by the physician in charge (either from ICU or internal medicine). In the TCZ branch, patients who received treatment with this drug were included. While in the conventional treatment (CT) branch, patients who did not receive the drug were included, either due to not being authorized by their welfare benefit plan, not being the drug available in the province, having improved their clinical status or died.
CT consisted of dexamethasone, oxygen therapy (by nasal cannula, reservoir mask or high-flow nasal cannula (HFNC)) and thromboprophylaxis.
The TCZ dose used was 8mg/kg/IV, maximum of 800mg. Thromboprophylaxis was performed with enoxaparin 40mg/d/SC in healthy weight patients, and 60mg/d/SC in overweight or obesity. Regarding treatment with corticosteroids, it was ordered in patients who presented hypoxemia, maximum of 10 days. The dose of dexamethasone used was 8mg/d/IV, while methylprednisolone was 2mg/kg for the first 5 days and then 1mg/kg for 5 more. The criterion for ordering methylprednisolone was the lack of clinical response to dexamethasone and the certainty that the patient would not receive TCZ. The indication of antibiotics was established by outpatient follow-up physician prior to hospitalization and suspended at the time of hospital admission. The most frequent were amoxicillin plus clavulanic acid and azithromycin.
Statistical analysisIt was carried out with the Epi Info 7.2 program. We used measures of central tendency, dispersion (95% CI), chi2 and Student's test, with significance criterion α error<5%, logistic regression model (entering variables with p<0.2), analysis with Cox regression, curves survival by the Kaplan Meier method.
OutcomesTo evaluate in-hospital mortality of patients admitted with COVID 19 comparing those who received treatment with TCZ and those who received CT and to evaluate the requirement of Intensive Care Unit (CCU) admission, invasive mechanical ventilation (IMV) and hospital stay between patients treated with TCZ versus those who received only CT.
ResultsOf the 204 patients admitted to the Institution with a diagnosis of SARS-COV2 infection, 90 (44%) were included in the study, 51 TCZ branch and 39 CT branch. 100% of the patients received treatment with systemic corticosteroids. The demographic and anthropometric characteristics of the population are described in Table 2. Regarding vaccination against SARS-COV2, none of the vaccinated patients had the complete two-dose schedule.
Comparative analysis between branches that used TCZ versus CT.
| Variable | TCZ 51 (56.7) | CT 39 (43.3) | p |
|---|---|---|---|
| Age (years) | 47.8 (12.4) | 48.6 (10.8) | 0.8 |
| Over 65 years | 5 (9.8) | 2 (5.1) | 0.4 |
| Male | 41 (83.4) | 33 (84.6) | 0.6 |
| Charlson | 1 (1.3) | 1.1 (1.2) | 0.5 |
| APACHE II | 5.6 (2.8) | 6.7 (3.1) | 0.06 |
| Comorbidities | 35 (68.6) | 31 (79.5) | 0.2 |
| HBP | 13 (25.5) | 19 (48.7) | 0.02 |
| Diabetes mellitus | 5 (9.8) | 8 (20.5) | 0.2 |
| Obesity | 26 (50.9) | 25 (64.1) | 0.2 |
| Vaccination SARS-Cov2 | 13 (25.5) | 10 (25.6) | 0.9 |
| Place of admission | |||
| Medical clinic | 50 (98) | 35 (89.7) | 0.08 |
| CCU | 1 (2) | 4 (10.3) | 0.09 |
| Days from onset of symptoms | 7.9 (2.3) | 8.1 (2.9) | 0.6 |
| Severity of COVID 19 at admission | |||
| Critical | 15 (29.4) | 11 (28.2) | 0.9 |
| Severe | 35 (68.6) | 26 (66.7) | 0.8 |
| CALL at admission | 8.5 (1.9) | 8.9 (2.2) | 0.4 |
| Need for CCU | 12 (23.5) | 14 (38.9) | 0.1 |
| Days in CCU | 8.7 (6.8) | 11.8 (8.5) | 0.3 |
| IMV | 4 (7.8) | 12 (30.8) | 0.005 |
| Days in IMV | 12.3 (9.9) | 12.4 (7.9) | 0.9 |
| Death | 1 (2) | 6 (15.8) | 0.02 |
| Hospital stay | 13(6.3) | 12.9 (7.1) | 0.7 |
| Imagenological evolution | |||
| Progression | 24 (61.5) | 23 (88.5) | 0.02 |
| Resolution | 8 (20.5) | 1 (4) | 0.06 |
| Pulmonary embolism | 3 (7.7) | 2(7.4) | 0.9 |
| HFNC in Medical Clinic | 28 (54.9) | 22(56.4) | 0.9 |
TCZ: tocilizumab, CT: convencional treatment, HBP: high blood pressure, CCU: critical care unit, IMV: invasive mechanical ventilation. HFNC: high-flow nasal cannula.
94.4% of the patients were admitted to Medical Clinic (MC) while 5.6% did so to the CCU. The days post symptoms onset (PSO) to hospitalization had a mean of 7.9 (± 2.6). The severity of COVID 19 on admission was severe in 67.8% and critical in 28.9%, while only 3.3% of the patients were admitted as moderate cases. 62.2% had previous emergency ward service consultation, with a median of 1 (0–4).
As regards to initial symptoms, the predominant symptoms were fever greater than 38°C (80%), dyspnea (71.1%), myalgia (74.4%) and cough (63.3%). Of those hospitalized, 37.8% added symptoms, with a mean of 6.9 (± 2.8) days, being dyspnea the most frequent (94.1%), followed by persistent fever (52.9%).
Regarding the evolution of hospitalized patients, 29.9% needed CCU, 17.8% required IMV, with a mean number of days of 12.4 (± 7.9). Among the patients who used IMV, 87.5% of them required prone. 7 patients died (7.9%). The average of hospital stay was 12.9 (6.6) days. At the time of the analysis, 4 patients were still hospitalized.
The most frequent complications were bacterial superinfection (18.9%), shock (6.7%), acute kidney injury (8.9%), hemodialysis requirement (2.2%), motor sequelae (10.2%), requirement of home oxygen therapy (16.1%), hyperglycemia due to corticosteroids (13.3%) and requirement of tracheostomy (4.4%).
A comparative analysis was performed between the branches that used TCZ vs TC (Table 2). Those patients who received TCZ had fewer comorbidities on admission and lower APACHE II score 48h after hospitalization, although the first point was not statistically significant. They required fewer CCU and, of the patients who needed their transfer, spent fewer days in this unit, although this was not statistically significant. However, it was statistically significant that the TCZ group required less IMV and less patients died. Hospital stay was not affected by the use of TCZ. Regarding the imaging evolution, the patients with TCZ showed less infiltrate progression in the control tomographies performed after 72h, than those who did not receive it, with statistically significant results.
The average number of days from to TCZ infusion was 11.1 (2.2). The mean from hospital admission to infusion was 3.2 (1.5) days. The mean number of days from the infusion to hospital discharge was 9.9 (6.3). The patient who died in the TCZ branch received it on the 15th day of symptoms. The only complication observed was the development of transaminitis, which occurred in 17.3% and had no other implications than the need for subsequent laboratory controls.
Regarding HFNC, they were used in 55.6% of the sample, observing more days of use for the TCZ branch 6 (±3) vs. 4.2 (±2.5) p 0.03, with a greater favorable response (85.7% vs. 63.6% p 0.07).
If we compare complications in both groups, the TCZ arm had less bacterial superinfection (6% vs. 31.6% p 0.002), shock (6% vs. 21% p 0.03), motor sequela (4% vs. 16.2% p 0.05) and oxygen therapy requirement at discharge (12.2% vs. 21.1% p 0.3).
Of the patients who required transfer to CCU, the first reason was the lack of availability of HFNC at MC, being in the TCZ branch 7 vs. 12 in the TC branch. Of the 7 patients in the TCZ arm, they all experienced HFNC placement, only 3 failed and required IMV. Only one died. It should be pointed out that one of them received the TCZ already intubated. Of the TC group, 10 patients used HFNC, with failure in 7 of them and death in 3. The remaining 2 required IMV and died. The second reason for CCU was the need for close monitoring of the HFNC in only 4 patients TCZ arm, and none of them failed or died.
A comparative analysis of inflammatory parameters of each branch was performed, where statistically significant improvement can be observed in them when tocilizumab is used (Table 3) and also considering the primary objective, univariate analysis of mortality was made (Table 4). The logistic regression did not obtain independent predictor variables of mortality (Table 5). The administration of TCZ was a protective factor against death with an RR 0.86 (0.74–0.99), not so for the transfer to CCU (RR 0.8; 0.61–1.12). The need for IMV doubled the risk of dying (RR 2.0, 1.23–3.42).
Comparative analysis of inflammatory parameters of each branch.
| Variable | TCZ | CT | p |
|---|---|---|---|
| CRP | |||
| Admission | 117.8 (78.9) | 114.6 (64.9) | 0.9 |
| 48h | 35.1 (43.4) | 83.6 (63.4) | 0.0007 |
| 5° day | 6.4 (4.8) | 63.2 (60.2) | 0.000 |
| 7° day | 3.6 (5.6) | 62.9 (77.6) | 0.0001 |
| Ferritin | |||
| Admission | 1594.5 (897.2) | 2043.5 (1335.8) | 0.06 |
| 48h | 2098.9 (1177.4) | 1964 (1014.7) | 0.7 |
| 5° day | 1904 (1015.3) | 2691.6 (1413.8) | 0.03 |
| 7° day | 1450.3 (838.2) | 2195.3 (1207.4) | 0.01 |
| IL-6 | |||
| Admission | 51.8 (56.2) | 54.4 (69.9) | 0.9 |
| 5°/7° day | 236.8 (400.8) | 90.9 (183.1) | 0.2 |
| LDH | |||
| Admission | 425.3 (224.5) | 469.1 (254.3) | 0.4 |
| 48h | 358.3 (171.6) | 488.2 (197.4) | 0.009 |
| 5° day | 338.5 (166.6) | 381.9 (182.3) | 0.3 |
| 7° day | 320.8 (153.1) | 447.9 (244.5) | 0.03 |
| D-dimer | |||
| Admission | 0.6 (1.2) | 0.4 (0.4) | 0.3 |
| 5° day | 1.7 (2.2) | 0.4 (0.3) | 0.02 |
| 7° day | 1.5 (2.1) | 1.2 (1.2) | 0.6 |
| Lymphocytes | |||
| Admission | 995.5 (397.4) | 1101.1 (472.8) | 0.3 |
| 48h | 1241.5 (592.9) | 979.6 (505.7) | 0.05 |
| 5° day | 1653.4 (873.1) | 1260.5 (972.7) | 0.02 |
| 7° day | 1534.8 (879.9) | 1376.4 (1004.7) | 0.3 |
| PaO2/FiO2ratio | |||
| Admission | 259.3 (99.4) | 245.6 (77.3) | 0.5 |
| 48h | 232.1 (117.7) | 190.1 (91.4) | 0.09 |
| 5° day | 258.8 (111.8) | 207.1 (95.7) | 0.04 |
| 7° day | 273.5 (118.3) | 238.9 (117.5) | 0.3 |
CRP: C reactive protein, LDH: lactate dehydrogenase.
Univariate analysis of mortality.
| Death 7 (7.9) | Alive 83 (92.1) | p | |
|---|---|---|---|
| Age (years) | 53 (4.3) | 47.9 (12.1) | 0.3 |
| Male | 6 (85.7) | 67 (82.7) | 0.8 |
| Charlson | 1.7 (1.4) | 1 (1.2) | 0.2 |
| APACHE II | 8.4 (2.1) | 5.8 (2.9) | 0.03 |
| Comorbidities | 7 (100) | 58 (71.6) | 0.1 |
| Obesity | 7 (100) | 44 (54.3) | 0.02 |
| HTA | 4 (57.1) | 28 (34.6) | 0.2 |
| Tocilizumab | 1 (14.3) | 49 (60.5) | 0.02 |
| Metilprednisolona | 0 | 17 (20.9) | 0.2 |
| Dexametasona | 7 (100) | 81 (100) | 1 |
| HFNC | 4 (57.1) | 44 (54.3) | 0.9 |
| IMV | 7 (100) | 7 (8.6) | 0.0000 |
| Need for CCU | 6 (100) | 18 (22.8) | 0.00005 |
| Hospital stay | 12.9 (7.4) | 13 (6.6) | 0.9 |
| Tocilizumab infussion after day 10 from onset of sympthoms | 1 (3) | 32 (65.3) | 0.5 |
HFNC: high-flow nasal cannula, IMV: invasive mechanical ventilation requirement, CCU: critical care unit.
Logistic regression of mortality.
| Odds ratio | 95% | CI | Coefficient | S.E. | Z-statistic | p-Value | |
|---|---|---|---|---|---|---|---|
| Comorbidities | 287,089.12 | 0.000 | >1.0E12 | 12.5675 | 398.9592 | 0.0315 | 0.974 |
| Tocilizumab | 0.1352 | 0.0148 | 1.2387 | −2.0007 | 1.1300 | −1.7705 | 0.076 |
| APACHE II | 1.2860 | 0.9540 | 1.7334 | 0.2515 | 0.1523 | 1.6511 | 0.098 |
| Constant | −15.8553 | 398.96 | −0.0397 | 0.968 |
In the Cox logistic regression, independent survival factors were the use of TCZ, the absence of obesity and the non-need for IMV, p 0.0000.
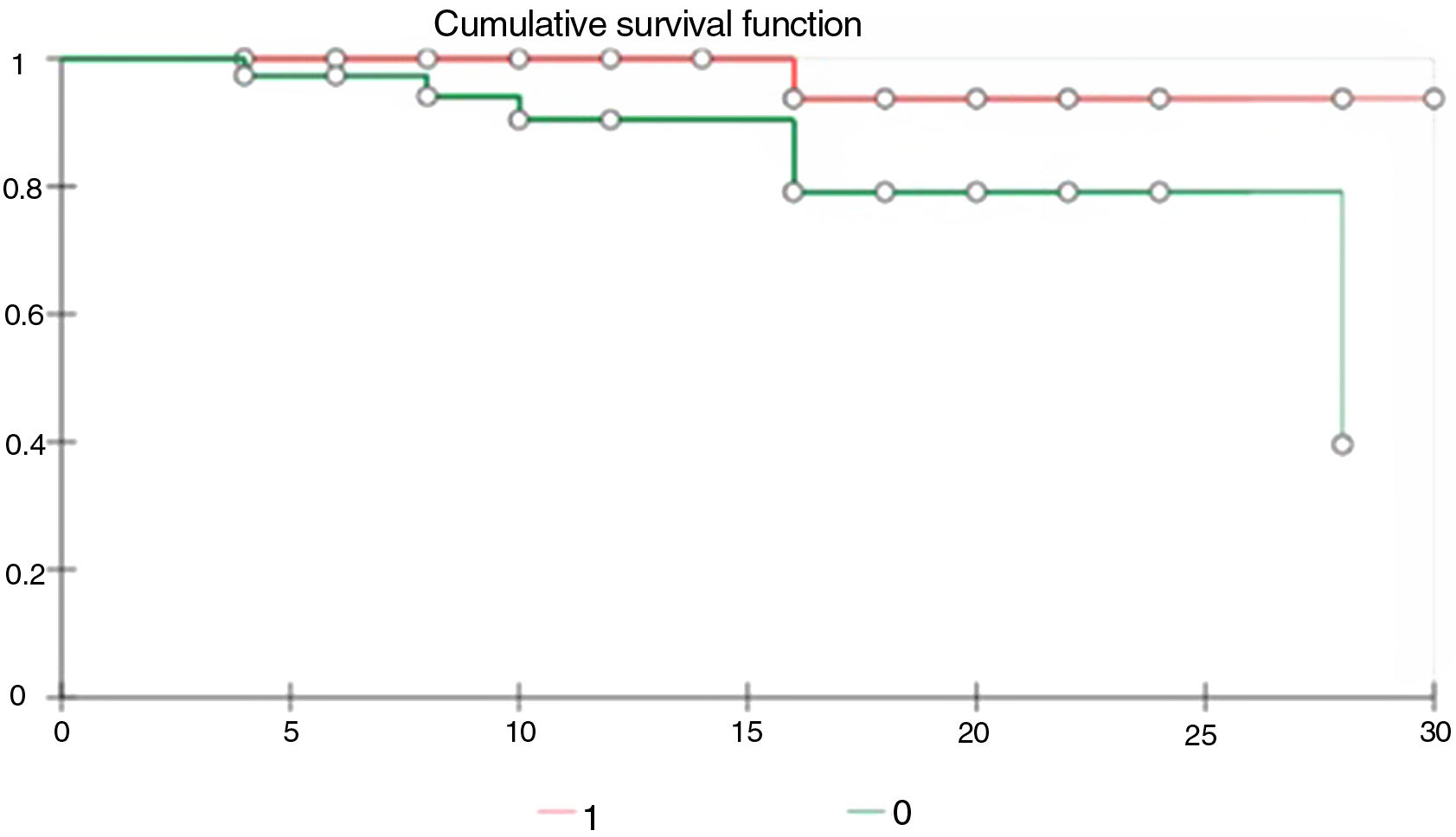
As we can observe in the Kaplan Meier curve of comparative survival, the patients who received TCZ had better survival than those who did not (Fig. 1).
DiscussionIn Argentina, the only recommended treatment to date is the use of systemic corticosteroids for severe and/or critical cases of COVID 19.14 However, the recent publication of the RECOVERY study in relation to the use of TCZ demonstrated a reduction in 28-day mortality and the need for IMV, in addition it describes the higher chances of hospital discharge within 28 days after admission.15,16 This new evidence added to the increase in the number of cases of critically ill patients and the limited number of beds in the CCU, led us to design an institutional protocol to reduce the impact on morbidity and mortality in our population.
We found out that in patients with severe or critical COVID-19 pneumoniae, combination treatment with IL-6 receptor antagonists such as TCZ and dexamethasone was effective compared to current standard of care treatment with corticoids.
Several papers that evaluate the use of TCZ associated with clinical improvement, inflammation markers, radiological evolution, and morbidity and mortality have been published.6,7,17–19
Multiple observational and ex vivo laboratory studies have shown that IL-6 is an important cytokine associated with the severity and mortality of COVID 19 disease. A recent genomic analysis in critically ill patients showed that genetic variants in the inflammatory pathway of this molecule are associated with a life-threatening disease. These observations support a therapeutic strategy to inhibit IL-6 pathways in patients with severe COVID 19.1,18,20
In concordance with our results, most of the reviewed publications showed a decrease in inflammation markers such as CRP and fibrinogen,7,18,21 but not IL-6 which was increased in most of them.1,2,6,7,17 In the study carried out in Miami by Vu C et al., a new increase in CRP was observed after day 10th, interpreted as secondary to the complete metabolization of TCZ.5
Regarding predictors of mortality in the use of TCZ, its administration within 12 days post symptoms onset22 and the decrease in inflammation markers such as CRP were evidenced as an independent factor.7,18,21 This was not verified in our study.
The EMPACTA trial showed that those patients who received TCZ were less likely to undergo mechanical ventilation or to die on day 28 than those who received placebo, although there were no statistically significant differences in overall mortality, the same as in a study conducted in Brooklyn, USA.23,24 These data were repeated in Italy.25 Despite this, in non-ventilated patients the prognosis did improve, which could justify its early use.1 In this same region, another study was carried out by Capra et al., where mortality rate highly decreased in those patients treated with TCZ.9 The same results were obtained in studies carried out in France by Laetitia A et al. and in Pakistan (the latter being of a single branch) added to the fact that those patients who received TCZ had a better tomographic evolution and a lower requirement for subsequent oxygen therapy.1,17 Mortality rate also decreased in a study by Van den Eyndea et al., although in this study the patients were also treated with antivirals and antibiotics in all branches, which favored the use of TCZ associated with corticosteroid therapy.3 The same combination managed to reduce deaths in Barcelona, although they could not define the best time to use TCZ.26 In a retrospective study carried out by the STOP-COVID research group, 4000 patients were evaluated and a lower risk of dying was found in those who received treatment with TCZ, as described by Huanga et al.10,27 In our study, those patients treated with TCZ required less transfer to the CCU, spent fewer days in this unit, required less IMV, and their mortality was lower.
The COVACTA trial, in which approximately 38% of patients had mechanical ventilation, showed no significant differences between the TCZ and placebo groups with respect to clinical status or mortality at day 28, although the time to hospital discharge was shorter with TCZ.28 A multicenter study carried out in Brazil, unlike our study, did not show differences regarding the IMV requirement or mortality at 15 and 28 days, nor did it decreased the time of oxygen therapy, but did show the length of hospital stay.2
As we mentioned before, the University of Washington published an improvement in inflammation markers, but could not demonstrate clear evidence about the possible clinical benefits or mortality with respect to the use of TCZ, although the population group that received TCZ was younger than the group control.7 The same results were published in a study conducted in Milan, Italy.8 The CONVINTOC study shares these conclusions regarding routine use in COVID 19, although it keeps the door open for its use in severe cases.8
In contrast to what was previously mentioned, in February 2021 a study was published where the use of TCZ resulted in higher mortality for those patients who were outside the CCU, in addition to requiring more IMV, and the hospital stay was longer, as well as the ventilation days.4
Although we have seen that there is a wide variety of results in the different published studies, predominantly better results have been observed in patients with severe COVID 19 pneumonia who received TCZ, based on retrospective observational cohort studies. Most showed a rapid reduction in inflammation markers in the laboratory, clinical improvement (using the presence of fever as the main reference), reduced use of oxygen support or mechanical ventilation, and mortality.19,29–31 In spite of that, these benefits have not been demonstrated in randomized controlled trials, so its use remains controversial.
In concordance with our study, a multicentre retrospective study in Italy called TESEO, included 544 patients, and compared treatment with hydroxychloroquine, azithromycin or antivirals vs adding TCZ. It showed that the use of TCZ in severe COVID 19 might reduce risk of mechanical ventilation and death. In this paper, patients treated with glucocorticoids were excluded.32
Regarding HFNC, we observed more days of use for the TCZ branch, with a greater favorable response. This allows us to think, correlating with the data on the need for IMV, that the use of TCZ would avoid IMV requirement, and increase the adaptation and response to equipment such as the HFNC.
One of the major limitations of our study is its retrospective and observational design, so the data collected should be viewed with caution. Despite this, in our population the indication for treatment was uniform and only compared combined treatment with TCZ and corticosteroids versus CT with corticosteroids. It should be pointed out that, since most of the published studies include antivirals, hydroxychloroquine, and azithromycin, among others, as conventional treatments.
ConclusionAlthough our work is based on a non-randomized study and the results should be interpreted with caution, it demonstrated that treatment with TCZ and systemic corticosteroids used in early stages of the exaggerated inflammatory response can reduce mortality.
Authors’ contribution- 1.
Conception and design of the manuscript: Cynthia Antonella Anci Alvarez, Solavallone Vanina, Romina Cardone.
- 2.
Data collection: Cynthia Antonella Anci Alvarez, Solavallone Vanina, Romina Cardone.
- 3.
Analysis and interpretation of the data: Cynthia Antonella Anci Alvarez, Solavallone Vanina, Romina Cardone.
- 4.
Drafting, review, approval of the submitted manuscript: Cynthia Antonella Anci Alvarez, Solavallone Vanina, Romina Cardone, Juan Manuel Orlando.
This research has not received specific aid from public sector agencies, commercial sector or non-profit entities.
Conflict of interestsNone of the authors declare any conflict of interest in relation to this publication.