Study of possible physiological events leading to progression of sepsis, i.e., inflammation, coagulation–anticoagulation balance and immunosuppression which have been addressed by new therapeutic targets for these metabolic pathways and thereby, evolution of drugs has better therapeutic potential than the traditional ones. Online database and printed material were searched using relevant keywords. Collected literature was scrutinized for related information with special reference to the objective. These drug molecules have either been approved for treatment of human subjects or these are under evaluation for therapeutic potential on human subjects or animal models. Surviving Sepsis Campaign guidelines include recommendations for recognition and early care, source diagnosis and treatment of infection, hemodynamic care, ventilation and additional therapeutic treatment recommendations. Advancements in these treatment strategies have reduced the mortality yet there is need for implementation of genomic and proteomic analysis techniques for better results. Smaller molecules should be preferred as therapeutic agents because of having less immunogenicity; smaller size is suitable for better tissue penetration.
Estudio de posibles eventos fisiológicos que conducen a la progresión de la sepsis, es decir, inflamación, equilibrio coagulación-anticoagulación e inmunosupresión, que han sido abordados por nuevas dianas terapéuticas para estas vías metabólicas y, por tanto, la evolución de los fármacos muestra un mejor potencial terapéutico que los fármacos tradicionales. Se realizaron búsquedas en bases de datos en línea y material impreso utilizando palabras clave relevantes. Se examinó la literatura recopilada en busca de información relacionada con especial referencia al objetivo. Estas moléculas de fármacos han sido aprobadas para el tratamiento de sujetos humanos o están bajo evaluación para determinar su potencial terapéutico en sujetos humanos o modelos animales. Las pautas de la campaña «Sobrevivir a la sepsis» incluyen recomendaciones para el reconocimiento y la atención temprana, el diagnóstico y el tratamiento de la infección, la atención hemodinámica, la ventilación y recomendaciones de tratamiento terapéutico adicional. Los avances en estas estrategias de tratamiento han reducido la mortalidad, pero es necesario implementar técnicas de análisis genómico y proteómico para obtener mejores resultados. Se deberían preferir las moléculas más pequeñas como agentes terapéuticos debido a que tienen menos inmunogenicidad: el tamaño más pequeño es adecuado para una mejor penetración en el tejido.
Sepsis has emerged as one of the leading causes of morbidity and mortality across the world. It is usually an immediate cause of death of critically ill patients in ICU (Intensive Care Unit). Cases of sepsis and septic shock have steadily been reported globally since its first consensus definition in 1991. Early identification and timely care has significant impact on reducing mortality. Microbiological analysis reveals most frequent bacteria is Gram-negative bacilli, e.g. Escherichia coli, Pseudomonas aeruginosa (most frequent cause of sepsis fatality), Klebsiella, and Gram-positive species, e.g. Staphylococcus aureus, Streptococcus species, Enterococcus species.1Candida species is the most frequent fungi causing invasive fungal infection.2 These microorganisms lead to sepsis of any part of the host body but most commonly on the skin, bowels, liver, kidneys, gall bladder and lungs.
Sepsis leads to hypovolemia, myocardial depression, increased metabolic rate and vasoregulatory perfusion abnormalities. These factors cause circulatory insufficiency which, in turn, leads to an imbalance between oxygen demand and oxygen delivery, resulting in tissue hypoxia and shock. As oxygen demand increases at the tissue level, oxygen extraction from hemoglobin increases, which is reflected into decrease in central venous oxygen saturations (ScvO2) or mixed venous oxygen saturations (SvO2). Once the limits of oxygen extraction have been reached, the tissues shift to anaerobic metabolism with subsequent production of lactate. This phase known as global tissue hypoxia, is an important transition of stages during sepsis and it may occur even in patients with normal vital signs.3 Central venous oxygen saturation and lactate levels are useful surrogates for monitoring severity of illness and for guiding treatment in septic patients.
Until third and recent redefinition of sepsis established in the year 2016, it was defined as systemic inflammatory response to infection categorized by specific clinical and laboratory findings and as the consequence of improper management, it may lead severe sepsis and septic shock. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference, 1991 defined sepsis as the systemic inflammatory response to infection having the presence of a suspected or confirmed infection accompanied by two or more systemic inflammatory response syndrome (SIRS) criteria (SIRS criteria as follows: temperature less than 36°C or more than 38°C, heart rate more than 90 beats per minute, respiratory rate more than 20 breaths per minute or partial pressure of carbon dioxide in arterial blood less than 32mm Hg, white blood cells count less than 4000mm3 or more than 12,000mm3, band more than 10%).4 There is absence of clinical imaging or biochemical indicators to indicate SIRS conditions. This leads to non-specificity of signs of SIRS criteria. Ultimately, there may occur significant discrepancy in the presentation of the incidence and mortality of sepsis in epidemiological studies. This demanded more specific definition to facilitate easier recognition of sepsis in day-to-day clinical practice.
The latest redefinition of sepsis (2016) highlights the host's inadequate response to infection. This refined definition is based on the pathobiology and pathophysiology of host's response to infection. Terms ‘SIRS’ and ‘severe sepsis’ were eliminated from the recent definition. Thus, as per the recent definition, sepsis is life-threatening organ failure caused by the host's inappropriate response to infection.5 Organ failure is considerable when there is a change in sequential, sepsis-related organ failure assessment (SOFA), where two points or more are associated with a hospital mortality rate greater than 10%.5 Septic shock is a sub-type of sepsis manifested by circulatory, cellular, metabolic instability associated with higher risk of death. Diagnostic criteria for septic shock include: hypotension requiring vasopressor therapy in order to maintain mean arterial pressure >65mm Hg and serum lactate levels greater than 2mmol/l after appropriate management of hypovalemia, the combination shows association with a hospital mortality rate of more than 40%.5
In relevance to avoid delay in treatment of patients who are placed outside the ICU, new simplified and fast version of SOFA scoring system has been designed called as quick SOFA (qSOFA), which is recommended for rapid diagnosis in outdoor patients and emergency hospital admissions for patients with suspected infection and sepsis.5 It assesses the patient's mental, cardiovascular and respiratory status; criterion for hypotension is systolic pressure <100mm Hg, for tachypnea respiratory rate >22 breaths per minute and Glasgow Coma scale (GCS) <15. qSOFA does not define sepsis but allows rapid identification of all patients at potential at risk of sepsis. It is easy to measure, does not inquire laboratory testing, can be performed repeatedly and quickly.
Surviving Sepsis CampaignDuring European Society of Intensive Care Medicine (ESICM) Annual Meeting 2002 at Barcelona, concept of Surviving Sepsis Campaign (SSC) was introduced with the “Barcelona Declaration”. SSC was initially administered by three organizations namely European Society of Intensive Care Medicine (ESICM), Society of Critical Care Medicine (SCCM) and International Sepsis Forum (ISF).6 The Barcelona Declaration demanded commitment from these three organizations to endeavor reduction in the mortality of sepsis by 25% within 5 years by improving recognition and treatment. The declaration urged governments and healthcare providers to recognize the growing burden of sepsis and commitment to provide adequate resources to combat it. The fourth updated SSC Campaign guidelines (published in 2021) include recommendations for recognition and early care, source diagnosis and treatment of infection, hemodynamic care, ventilation and additional therapeutic treatment recommendations (use of programs and tools like qSOFA, National Early Warning Result (NEWS), and Modified Early Warning Result (MEWS) to improve care, timely recognition of acutely ill and high risk patients).7 qSOFA is not recommended as the only method for recognizing sepsis and septic shock and it must be considered with SIRS, NEWS or MEWS. MEWS helps to improve quality and safety in patient care (five physiological parameters are measured: respiratory rate, systolic blood pressure, heart rate, level of consciousness and body temperature). NEWS is a scoring system used for physiological measurements routinely recorded next to the patient's bed and its aim is to identify acutely ill patients, including those with sepsis (six physiological parameters are measured and values from 0 to 3 are evaluated for these parameters: respiratory frequency, oxygen saturation, systolic blood pressure, pulse rate, neurological level of consciousness and body temperature).
MethodologySearch strategyWe searched for relevant studies by two means: (a) by online database searching using different online search engines and (b) from books, printed journals, government reports available in the Library of National Centre for Disease Control, Varanasi, India.
Database searchingElectronic databases namely, PubMed (http://www.ncbi.nlm.nih.gov/pubmed), ISI Web of Science (http://wokinfo.com/), Wolters Kluwer Health (http://www.wolterskluwer.com/Products/Health/Pages/default.aspx) and World Health Organization library database (http://www.who.int/library/databases/en/) were searched applying different keyword terms and Boolean operators. Our search was performed until 31st December 2023.
Inclusion and exclusion criteria for selectionRestriction was set to include only those studies which were carried out since the year 1991 and had information globally. No restriction was set for the language of the publication. Only original articles, technical and brief reports were included in this review. Abstracts without published articles were excluded. Bibliography from selected original articles was also screened for additional articles relevant to our study objective.
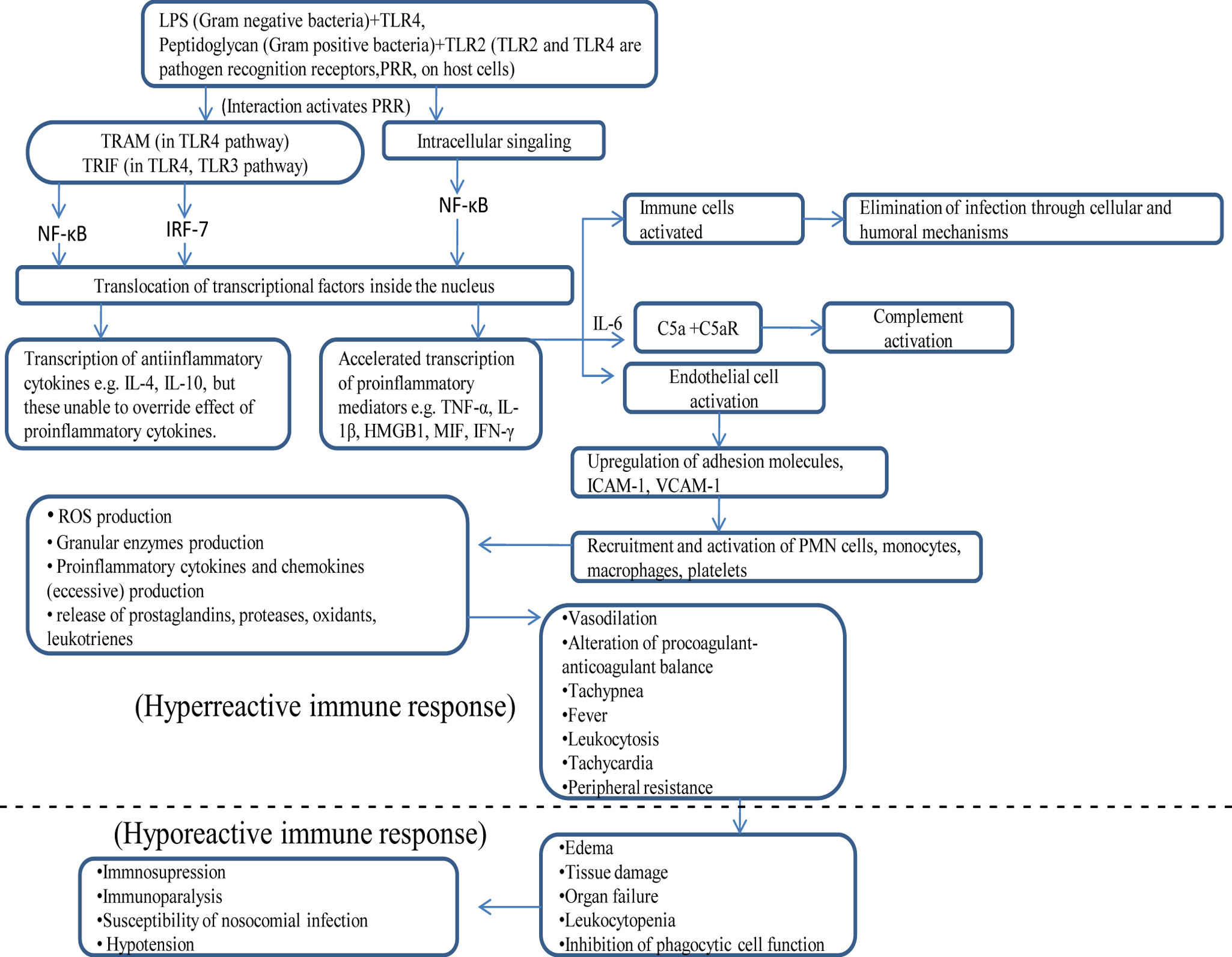
Immunological events during sepsis and their possible role in its progressionInflammation in early sepsisPattern-recognition receptor on the membrane of host cells recognizes cell wall derived pathogen-associated microbial patterns (PAMPs) in bacteria, fungi and viruses (e.g. TLR2 recognizes peptidoglycan of Gram-positive bacteria, and TLR4 recognizes lipopolysaccharide of Gram-negative bacteria). This interaction leads to activation of cytosolic nuclear factor κB (NF-κB) which translocates from the cytoplasm to nucleus and binds to transcription initiation sites. This accelerates transcription of pro-inflammatory cytokines e.g. TNF-α, IL-1β, and anti-inflammatory cytokines e.g. IL-10.8 IL-10 inactivates macrophages.9 Pro-inflammatory cytokines e.g. TNF-α, IFN-γ and IL-6 induce expression of adhesion molecules in endothelial cells.10 It facilitates binding of neutrophils, monocytes, macrophages and platelets to the endothelial cells. In addition to kill invading microorganisms, these effector cells release mediators like proteases, oxidants, prostaglandins and leukotrienes. These mediators injure endothelial cells causing increased permeability, vasodilation, and alteration of the procoagulant-anticoagulant balance. Sepsis-induced increased activity of inducible nitric oxide synthase (iNOS) leads to the increased synthesis and release of a potent vasodilator nitric oxide from the endothelial cells, which causes further vasodilation. The increased permeability of endothelium leads to the flow of protein-rich edema fluid into lungs and other tissues as well.
Role of HMGB1 in inflammation during sepsisHigh mobility group box-1 protein (HMGB1) is a non-histone nuclear and cytoplasmic protein. On binding with DNA it regulates its repair, recombination and transcription.11 Inflammatory stimulus (e.g. LPS) contributed by pathogen induces macrophages to produce HMGB1. It further activates phagocytic cells to produce pro-inflammatory cytokines (e.g. TNF-α, IL-1α, IL-1β, IL-6) and chemokines (e.g. MIP-1α). HMGB1 acts on endothelial cells through binding to RAGE over the surface of these cells and elicits JNK, ERK 1/2 and p38 MAPK kinases mediated intracellular signaling. This results in translocation of NF-κβ and SP-1 transcription factors inside nucleus where these results in exacerbated expression of pro-inflammatory cytokines and chemokines, PAI-1, tPA, RAGE, and adhesion molecules namely VCAM-1, ICAM-1. So, HMGB1 regulates fibrinolysis (through tPA expression) and more neutrophil recruitment through adhesion molecules.12,13 It enhances activity of intrinsic nitric oxide synthase (iNOS) causing heightened nitric oxide production. This leads to enhanced vascular permeability and bacterial translocation through the gut barrier. Higher concentrations of HMGB1 leads to disruption of enterocyte stability and can lead to acute lung injury.
MIF in sepsisMIF (macrophage migration inhibitory factor) is produced during early stage of sepsis by macrophages, T cells and pituitary cells in response to stimulation by cytokines (IFN-γ, TNF-α), glucocorticoids, and lipopolysaccharide of Gram-negative bacteria respectively.14,15 MIF further induces upregulated expression of TLR4 receptors over macrophages in response to lipopolysaccharide (effective signaling mode NF-κβ dependent). It also exacerbates the production of pro-inflammatory cytokines and chemokines by macrophages. T cell, on being activated by MIF, leads to production of antibody and cytokines (e.g. IL-2, IFN-γ). It is well known fact that glucocorticoids induce macrophage and T cell inactivation. Researchers have now reported that MIF overrides this activity of glucocorticoids and thereby, restoring the active state of macrophage and T cells.
Coagulation process during sepsisEndothelial cells being activated during sepsis lead to increased expression of tissue factor. Tissue factor activates coagulation cascade factor Va which in turn activates factor VIIIa. VIIIa leads to formation of thrombin-α required for the conversion of fibrinogen to fibrin. Fibrin binds to platelets and this complex adhere to endothelial cells forming microvascular thrombi. Microvascular thrombi amplify the severity of injury by releasing mediators and by microvascular obstruction which ultimately leads to distal ischemia and tissue hypoxia. Antithrombin III, tissue factor-pathway inhibitor (TFPI), natural coagulants (for example, protein C and its cofactor protein S) enhance fibrinolysis and remove microthrombi. Protein C forms complex with protein S. This activated protein C molecule proteolytically deactivates factors Va and VIIIa and inhibits the synthesis of plasminogen activator inhibitor 1(PAI-1).16 PAI-1 inhibits plasminogen formation.17 Plasminogen gets converted to active plasmin. Plasmin degrades the fibrin formed (fibrinolysis). In contrast, sepsis decreases levels of protein C, protein S, antithrombin III and TFPI, thereby inhibiting the natural anticoagulatory pathway of the patient.
Immunosuppression in sepsisImmunosuppression, loss of delayed hypersensitivity, inability to clear infection and predisposition to nosocomial infections are the characteristics often consistent with the sepsis. Initially, increment of inflammatory molecules occur, but later there is shifting toward an anti-inflammatory immunosuppressive response with the persistence of the sepsis. Studies on ex vivo lipopolysaccharides stimulation of monocytes obtained from patients with sepsis and healthy controls, indicates lower expression of pro-inflammatory cytokines in the former reflecting the immunosuppressive state of the patient with sepsis.18 Major mechanisms leading to sepsis are mentioned below:
- i.
Shifting of CD4+ T cells to secrete anti-inflammatory cytokines: Activated CD4+ T cells are programmed to secrete either pro-inflammatory cytokines e.g. TNF-α, IL-2, IFN-γ (TH1 cells) or anti-inflammatory cytokines e.g. IL-4, IL-10 (TH2 cells).19 The factors responsible for determination of fate of CD4+ T cells are not clearly understood but studies showed that these are influenced by type of pathogen, size of inoculums, and site of infection. Accelerated anti-inflammatory cytokines production leads to immunosuppressive state of the patient.
- ii.
Unresponsiveness of immune cells to antigen: This state is referred as “anergy”. T cells lost their capability to proliferate or cytokine secretion in response to an antigen. Defective cytokine secretion leads to higher mortality rate. It has been reported that sepsis-induced anergic state of immune cells may be triggered by apoptotic cell death. Gastrointestinal epithelial cells and lymphocyte cells undergoes apoptotic cell death during sepsis. When macrophages or dendritic cells ingest either apoptotic cells or necrotic cells, these induce anti-inflammatory cytokine profile (TH2)/anergy or inflammatory cytokine profile (TH1) and enhancement of antimicrobial defense respectively. Thus, the type of cell death determines the immunologic function of surviving immune cells. It has been proposed that cellular stress induces endogenous release of glucocorticoids inside the lymphocytes, which results in its apoptosis.
Apoptosis of lymphocytes in the Peyer's patches of the small bowel significantly contributes to the immunosuppressiveness of the patient. Although new insights to the potential mechanism of immune cells apoptosis still needs to be unveiled.
- iii.
Apoptosis induces loss of cells of adaptive immune system: During sepsis, apoptosis induced decreased concentration of CD4+ cells, B cells and follicular dendritic cells have been reported.20 Although decrease of population of CD8+ cells, natural killer cells, macrophages have not been clearly reported during sepsis. The loss of these cells becomes more important because a huge clonal expansion of the lymphocytes might be expected during severe sepsis. Lowering the rate of antibody production, macrophage activation, and antigen presentation due to the loss of B cells, CD4+ T cells and dendritic cells respectively weaken the capability of adaptive immune system of the host to fight against pathogenic invasion. Studies on the prevention of apoptosis of lymphocytes (e.g. targeted adenovirus-induced expression of IL-10 which further lowers the thymic apoptosis) may result in improvement of the survival of the host.
Early stage of sepsis leads to complement activation resulting in production of C5a serum proteins.21 C5aR (receptor for C5a) is distributed on mast cells, basophils, granulocytes, monocytes, macrophages, platelets, and endothelial cells of the host. IL-6 produced during early phase of sepsis, upregulates expression of C5aR on these cells, leads to escalated response of these cells to C5a. C5aR gets activated on binding with its ligand C5a and leads to degranulation of mast cells and basophils. C5a is a strong chemoattractant of neutrophils and on contact leads to reinternalization of C5aR inside neutrophils. It results in suppression of innate immune functions such as phagocytosis, chemotaxis, and H2O2 production. Increased expression of C5aR on membrane of thymocytes during the initial stages of sepsis leads to the apoptotic cellular mortality of these cells. Other immunological activities of C5a molecule during sepsis consists of increased vascular permeability of endothelial cells and, increased ROS production in macrophages and release of granular enzymes. In the presence of antigen (lipopolysaccharide) C5a effects endothelial cells, epithelial cells, and macrophages synergistically leading to further accelerated production of pro-inflammatory cytokines (e.g. TNF-α, IL-6, IL-1) and chemokines (e.g. MIP-2, CINC, MCP-1, KC). Summary of possible physiological events that may lead to the progression from early sepsis to septic shock has been illustrated in Fig. 1.
ConclusionAlthough studies revealing molecular events that lead to immunosuppressiveness have been elucidated significantly, still the exact mechanism behind suppressed immune status of patient with sepsis is still needed to be unveiled since the choice of enhancing or suppressing the immune system of the patient may be a therapy against sepsis depending upon degree of illness and type of pathogenic organism. Genomic and proteomic analysis of multiple pathways involved in sepsis will be advantageous to express the molecules and mediators mostly involved in inflammation and immunosuppressiveness as well as to monitor the state of illness of the patient with sepsis at a particular time. In addition, information illustrated by these molecular analyses will address appropriate therapy against particular stage of sepsis. Application of molecular detection techniques such as real time-PCR will be able to detect the type of pathogenic bacteria involved thereby, suggesting the development of appropriate monoclonal antibodies against the pathogen, and antagonists against endotoxins and superantigens.
Proper blood glucose management, treatment of cellular oxygen deficit, coagulation cascade blockers e.g. recombinant activated protein C and administration of proper antimicrobials are the major therapeutic strategies applied by clinicians to manage sepsis. One of the possible reasons behind the failure of most of the expensive, time consuming clinical studies to establish transparent association between new therapeutic strategies and survival of patients with sepsis is that these studies mostly involved mixed population rather than concentrating on a specific subgroup of sepsis. In addition, smaller molecules should be preferred as therapeutic agents because of having less immunogenicity, smaller size, suitability for better tissue penetration. In brief, advancements in modern treatment strategies will represent sophisticated menu for treatment of sepsis and physicians will be benefited up to a greater extent.
FundingNone declared.
Authors’ contributionsAD, PKS, AKY and VP were involved in the conception and design of the study, acquisition of data. AD performed analysis and interpretation of data. AD, RR drafted the article. VKG revised for important intellectual content. AKY, ZA, VKG and AG approved final version of the article.
Competing interestThere are no conflicts of interest for any author.