Dysphagia, defined as difficulty in swallowing, significantly impacts critically ill patients, especially those in intensive care units (ICUs). It can arise from neuromuscular disorders or mechanical obstructions, often exacerbated by prolonged mechanical ventilation and other medical interventions. Dysphagia poses risks such as aspiration pneumonia, malnutrition, and prolonged hospital stays. Early detection and intervention are crucial. A comprehensive literature search was conducted using PubMed, SCOPUS, and SPRINGER databases from December 2023 to February 2024. Search terms included “screening,” “dysphagia,” “ICU,” “ultrasonography,” “swallowing function,” and “deglutition disorders.” The search yielded 43 relevant articles, which were subjected to narrative analysis. Traditional dysphagia assessment methods include bedside evaluations, swallow tests, and instrumental strategies like video fluoroscopic swallowing study (VFSS) and fiberoptic endoscopic evaluation of swallowing (FEES). Ultrasonography (US) emerges as a promising, non-invasive alternative for evaluating swallowing function. US can assess tongue and hyolaryngeal movements, and upper esophageal sphincter function, offering real-time feedback and aiding in diagnosis and therapeutic planning. Despite its benefits, US lacks standardized protocols and comprehensive diagnostic criteria, necessitating further research. Ultrasonography shows significant potential in evaluating and managing dysphagia in critically ill patients due to its non-invasive nature and real-time feedback capabilities. However, variations in techniques and the need for standardized diagnostic criteria present challenges. Future research should focus on standardizing ultrasound parameters and refining diagnostic criteria to enhance its reliability and accuracy. Despite these challenges, ultrasonography remains a promising tool for dysphagia assessment and intervention.
La disfagia, definida como la dificultad para deglutir, impacta significativamente a los pacientes críticamente enfermos, especialmente a aquellos en las unidades de cuidados intensivos (UCI). Puede surgir de trastornos neuromusculares u obstrucciones mecánicas, a menudo exacerbadas por la ventilación mecánica prolongada y otras intervenciones médicas. La disfagia conlleva riesgos como neumonía por aspiración, desnutrición y estancias hospitalarias prolongadas. La detección e intervención tempranas son cruciales. Se realizó una búsqueda exhaustiva de la literatura utilizando las bases de datos PubMed, SCOPUS y SPRINGER desde diciembre de 2023 hasta febrero de 2024. Los términos de búsqueda incluyeron «screening», «dysphagia», «ICU», «ultrasonography», «swallowing function» y «deglutition disorders». La búsqueda arrojó 43 artículos relevantes, los cuales fueron sometidos a análisis narrativo. Los métodos tradicionales de evaluación de la disfagia incluyen evaluaciones a pie de cama, pruebas de deglución y estrategias instrumentales como el estudio videofluoroscópico de la deglución (VFSS) y la evaluación endoscópica de la deglución con fibra óptica (FEES). El ultrasonido (US) surge como una alternativa prometedora y no invasiva para evaluar la función de la deglución. El US puede evaluar los movimientos de la lengua y el hioides, y la función del esfínter esofágico superior, ofreciendo retroalimentación en tiempo real, y ayudando en el diagnóstico y en la planificación terapéutica. A pesar de sus beneficios, el US carece de protocolos estandarizados y criterios diagnósticos completos, lo que requiere más investigación. La ecografía muestra un potencial significativo en la evaluación y en el manejo de la disfagia en los pacientes críticamente enfermos debido a su naturaleza no invasiva y capacidades de retroalimentación en tiempo real. Sin embargo, las variaciones en las técnicas y la necesidad de criterios diagnósticos estandarizados presentan desafíos. La investigación futura debe centrarse en estandarizar los parámetros de ultrasonido y refinar los criterios diagnósticos para mejorar su fiabilidad y precisión. A pesar de estos desafíos, la ecografía continua siendo una herramienta prometedora para la evaluación e intervención de la disfagia.
Dysphagia is the “difficulty in swallowing which may result from neuromuscular disorder or mechanical obstruction”; it is also noted that this entity can be classified into oropharyngeal dysphagia and esophageal dysphagia.1 Additionally, authors such as Regan et al. (2019)2 describe an iatrogenic etiology as a collateral result of a procedure or intervention, without the aim of generating difficulty in swallowing, such as dysphagia secondary to prolonged mechanical ventilation (MV), radiation, among others. Similarly, dysphagia interferes with the effective and safe transit of the bolus (liquid or solid) from the oral cavity to the stomach; Cámpora and Falduti (2012)3 propose functionally characterizing dysfunction to guide both diagnosis and intervention in dysphagia in different phases: oral preparatory, oral, pharyngeal, esophageal, oropharyngeal, and pharyngoesophageal.
The incidence of this swallowing alteration was recently described in a prospective study that evaluated the incidence and evolution of dysphagia after mechanical ventilation (DYNAMCS study), Schefold et al. described a 12.4% rate among ICU patients.4 Additionally, they found a consistent significance HR for 28-day mortality for patients with dysphagia (even when adjusting for severity and days of mechanical ventilation). These findings are consistent with other reports in the literature.5,6 This entity is a concern in hospitalized patients on intensive care units (ICUs) with screening strategies, early evaluation and diagnostic processes needed.
The burden of dysphagia is considered high; in a population-based study, it was described that it affected 3.0% of all adult US inpatients who had a significantly longer hospital length of stay, higher costs, need of post-acute care facility and inpatient mortality.7 This impact represents risks for different outcomes such as: aspiration, aspiration-induced pneumonia, delayed resumption of oral intake, malnutrition, dehydration, decreased quality of life, prolonged ICU and/or hospital length of stay and increased morbidity and mortality.6 It has been estimated that dysphagia in the ICU increases 90-day mortality by 9.2% and one-year mortality by 25%. Additionally, these complications may persist in up to one-third of patients for 5 years, resulting in a considerable burden of morbidity. Despite the clarity of this issue, there is still no standardization for early detection and management. In a study that collected data from 69 countries, only 28% of ICUs had a protocol related to dysphagia. The importance of this becomes evident when considering that early identification of patients with dysphagia should reduce the risk of the before mentioned complications, improving patient quality of life and clinical outcomes.
Moreover, studies show that 36% of patients with post-extubation dysphagia have silent aspiration due to altered glottic and subglottic sensation8 emphasizing the need for adequate evaluation with sensitive and cost-effective methods to determine the risk of aspiration before initiating oral intake.
The challenge begins with screening strategies and early protocols to identify the presence of dysphagia once the patient is in the post-extubation period. However, it appears that awareness of this evaluation is not widespread.9 In this scenario, it is necessary to create strategies and teams for the identification and prompt treatment. With this in mind, the main objective of this narrative review is to present the use of ultrasonography in the evaluation of dysphagia in critically ill patients.
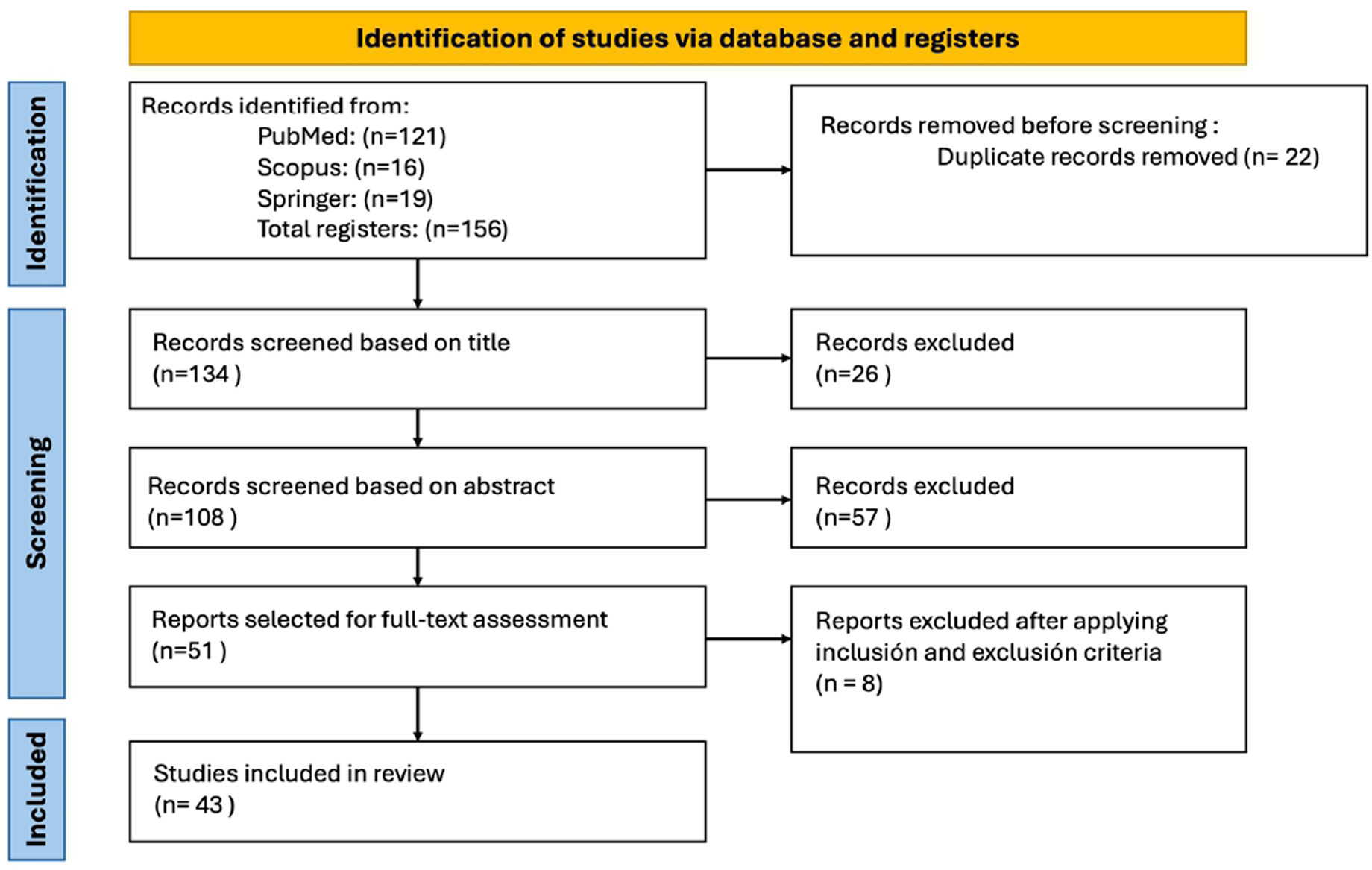
MethodsA literature search was carried out to compile studies, which were then subjected to a narrative analysis. The search was performed between December 2023 and February 2024 using the PubMed, SCOPUS, and SPRINGER databases. Boolean operators “AND” and “OR” were used to construct the search strategy with the following MeSH terms: “screening”, “dysphagia”, “ICU”, “ultrasonography”, “swallowing function”, and “deglutition disorders”. The search for studies in the different electronic databases was conducted independently by two researchers using the same terms and search strategy. This approach resulted in duplicates, which were removed during the subsequent step.
Each researcher carried out the study selection process based on predefined criteria. The inclusion criteria were that the studies be written in English or Spanish and that the articles were reviewed to contextualize our findings. An initial evaluation was performed based on the title, followed by reading the abstract. Finally, by reading the full text, a subset of 43 final articles were included in the narrative review (see Fig. 1, flowchart). The researchers compared annotations and significant findings, and these were organized by topic.
ResultsEvaluation of dysphagia in ICUAs mentioned by Spronk et al., few studies or guidelines for prevention, assessment, evaluation, and/or treatment of dysphagia for ICU patients are available.10 In a multicenter survey, they found that dysphagia is an ICU recognized problem and should have a standardized evaluation.10 It is also mentioned that critical care practitioners consider that a protocolized evaluation should be carried out on patients who required invasive mechanical ventilation by orotracheal tube for at least 48h. What concerns about the survey results is the few reports on standardized processes to address dysphagia, as well as the fact that a large part of them occur outside the intensive care unit.10
Assessment methods used to detect oropharyngeal dysphagia in ICU6,11:
- •
Self-report.
- •
Bedside swallowing evaluation.
- •
Water swallow test.
- •
Gugging Swallowing Screen.
- •
Volume viscosity swallow test.
- •
EVANS test.
- •
The Mann Assessment of Swallowing Ability.
- •
Video fluoroscopic swallowing study.
- •
Fiberoptic endoscopic evaluation.
Bedside swallowing assessment refers to the evaluation of swallowing function conducted by speech-language pathologists. Detailed information regarding the structure and function of oropharyngeal musculature is obtained through clinical history and physical examination. It begins with an assessment of movement, lip seal, tongue strength, dentition, and vocal quality. Additionally, cranial nerve assessment tests are performed.11 In addition, the evaluation of the postural (musculoskeletal), respiratory, phonatory, cognitive and linguistic component is taken into account in relation to the oro-motor and oropharyngeal processes of safe and effective swallowing.
Subsequently, validated tests are used, such as providing foods of five different standard consistencies (different liquids and solids), ranging from boluses with lower to higher risk of aspiration (lower to higher), or the ice chip protocol is used when generally the patient comes in nothing orally, thick liquids such as nectar, honey, clear or light, pureed solids, or in original consistency as appropriate.
As the boluses are administered, the expert observes five predefined signs of aspiration after each bolus: presence of coughing, throat clearing, change in vocal quality, wet vocal sounds during breathing, and presence of stridor11 in addition to altered vital signs, specifically oxygen saturation. If any of these signs are positive, it is recorded as a test consistent with aspiration. In addition to taking into account other clinical signs such as: lingual activity in the preparation of the bolus, anterior effusion (in the labial area), physiology of the masticatory system, hyolaryngeal activity, muscular and postural compensations, residues in the oral cavity after swallowing.11
3-Ounce swallow testFollowing the bedside swallowing assessment, speech-language pathology specialists utilize the 3-ounce swallow test. Patients are instructed to drink 3 ounces or 90cm3 of water without interruption, while the specialist evaluates various aspects such as: presence of coughing, sensation/perception of wet voice or if the patient cannot complete the test.12
Modified blue dye test (MBDT)The MBDT, also known as the modified Evans blue dye test, is an assessment for dysphagia specifically designed for critically ill patients with tracheostomies. It involves administering foods or liquids infused with a solution of blue dye to the patients. Subsequently, the presence of blue discoloration in the patient's respiratory secretions is evaluated, which may indicate pulmonary aspiration in patients with dysphagia.13
Volume viscosity swallow test (VVST)The VVST, also known as the volume viscosity swallow test assisted by the speech-language pathology specialists, progresses from the viscosity of the liquid consistency of nectar (295mPas), liquid (21mPas) and pudding (3682mPas), in increasing order with boluses of (5, 10 and 20mL). It begins with the nectar consistency and the progression of the volumes is tested. If signs of aspiration and/or penetration are identified, it is advanced to the pudding, and if not, it is advanced to the clear liquid. In addition to identifying aspiration and/or penetration characteristics, the test allows us to know the safest and most effective volume and viscosity for the patient.14
EAT-10The Eating Assessment Tool-10 (EAT-10) is a questionnaire designed to identify patients at high risk of swallowing disorders. Studies demonstrate its significant utility in screening for possible dysphagia in patients with issues in the oropharyngeal and esophageal phases.15
Through a self-assessment scale, patients are asked 10 questions about their eating habits and potential difficulties while consuming solid or liquid foods, among others. Based on the level of difficulty reported by the patient, each question accumulates a certain number of points, with a maximum total score of 40. A higher overall score indicates greater severity of symptoms.
Gugging Swallowing Screen (GUSS)The Gugging Swallowing Screen (GUSS) is a reliable and sensitive test for detecting dysphagia, consisting of two parts: a preliminary assessment, also known as the indirect swallowing test, and a direct swallowing part.16 The indirect swallowing part involves assessing the patient's ability to produce coughing and swallow saliva without voice changes or drooling. If this test is successful and a score higher than 5 is obtained, the direct swallowing part is continued. In the direct part, swallowing is evaluated by administering boluses of different textures, and the patient is assessed for signs of aspiration such as delayed or absent swallowing, coughing, drooling, or voice changes.14 Scores range between 20, indicating minimal risk of aspiration, and 0, indicating high risk of aspiration.
Instrumental strategies available for the identification of dysphagia in patientsFiberoptic endoscopic evaluation of swallowing (FEES)FEES is an instrumental strategy where a fiberoptic endoscope is inserted through the nose to conduct a comprehensive evaluation of patients’ swallowing. This technique allows visualization of the movement of anatomical structures in the pharynx and larynx, such as the epiglottis, vocal cords, and laryngeal muscles.
Additionally, this strategy assesses the safety of swallowing by asking the patient to swallow a food bolus, evaluating the movement and sensation of the bolus, as well as the management of the patient's secretions during the pharyngeal stages of swallowing.17 If the patient exhibits any reflex in response to the food stimulus, it is also recorded in real time, allowing the expert to obtain the necessary findings for diagnosis and therapeutic planning.
Video fluoroscopic swallowing study (VFSS)VFSS is a study which uses real-time X-ray fluoroscopy to evaluate the swallowing function of patients by observing the movements of different structures such as the tongue, pharynx, epiglottis, and esophagus to identify findings that demonstrate either a good swallowing reflex or the presence of structural and functional abnormalities while the patient ingests a variety of foods, liquids, and contrast materials.18
This technique allows for both qualitative and quantitative assessment of swallowing function. In qualitative assessment, the expert radiologist can observe motor functions such as palate movement, hyolaryngeal complex, muscle opening, and presence of residue in different structures. On the other hand, in quantitative assessment, this technique provides real-time images of swallowing and upper airway function with temporal and kinematic parameters.19
Temporal parameters19- •
Oral transit time
- •
Duration of soft palate elevation
- •
Duration of the hyoid bone at rest
- •
Duration of hyoid bone at movement
- •
Duration of upper esophageal sphincter opening
- •
Swallow reaction time
- •
Pharyngeal transit time
- •
Duration of closure of the laryngeal vestibule
- •
Superior movement of the hyoid bone
- •
Anterior movement of the hyoid bone
- •
Diameter of upper esophageal sphincter opening
- •
Pharyngeal area at rest
The PAS score, Penetration-Aspiration Scale by Rosenbek, is a scale that provides a severity grade based on an overall assessment of a patient's swallowing performance.20 It is important to note that it does not constitute a diagnostic method per se but rather a pathophysiological description of the presentation of dysphagia that has already been diagnosed. It also allows for the measurement of progression parameters. See Table 1.
PAS score.
| Punctuation | Description | |
|---|---|---|
| Penetration | 1 | The material does not enter to the airway |
| 2 | The material enters the airway, above vocal cords and is expelled | |
| 3 | The material enters the airway, above vocal cords and is not expelled | |
| 4 | The material enters the airway, has contact with vocal cords and is expelled | |
| 5 | The material enters the airway, has contact with vocal cords and is not expelled | |
| Aspiration | 6 | The material enters the airway, exceeds the level of the vocal cords, and is expelled into the larynx or out of the airway |
| 7 | The material enters the airway, exceeds the level of the vocal cords, and is not expelled into the larynx or out of the airway | |
| 8 | The material enters the airway, exceeds the level of the vocal cords, and is not expelled, there is not cough | |
Adapted from Alkhuwaiter et al.20
The diagnostic yields of different dysphagia tests have been reported compared to videofluoroscopy. FEES has reported different sensitivities depending on the degree of dysphagia, ranging between 87.5 and 90.9%, which increases with higher PAS scores.21 However, other studies have shown that FEES has high sensitivity (89%) for detecting food penetration problems but very low (40%) for aspiration. Moreover, it seems that FEES has a very high predictive value for the latter attribute (94%).22 On the other hand, the performance of EAT-10 varies depending on whether it evaluates its ability to detect the risk of dysphagia or the presence of dysphagia itself. EAT-10 has a sensitivity of 71.42% and specificity of 45.45% in detecting the risk of dysphagia, and regarding the detection of dysphagia, this questionnaire has sensitivity and specificity of 47.61% and 12.5%, respectively.23 GUSS was found to be the most sensitive test of all and most useful for dysphagia screening (sensitivity 97–100% and positive predictive value 100%); however, its specificity is only 50%.24
Which method is the gold standard?VFSS is the gold standard as described in the literature.4–6 It allows for the capture of fluoroscopic images displayed on a monitor while the patient ingests a radiopaque bolus. This allows a precise evaluation not only of the morphological characteristics but also, and above all, of the biomechanics of swallowing, including its three phases: oral, pharyngeal and esophageal. VFSS has advantages (it is non-invasive like FEES, evaluates all stages including gastric emptying and laryngeal ascent) and disadvantages (exposure to radiation, requires equipment displacement typically). Although over the years various instrumental investigations have been used alongside VFSS, such as video endoscopy, oropharyngeal scintigraphy, and manometry, which have been widely used in studies on swallowing, VFSS continues to play a crucial role in obtaining more detailed diagnostic data on swallowing disorders.25,26
Ultrasound for the evaluation of dysphagiaUltrasonography has been used to study dysphagia in patients with these neurologic conditions: Parkinson's disease, muscle dystrophy, amyotrophic lateral sclerosis, and stroke. The outcomes of ultrasonography included the thickness of swallowing muscles (e.g., tongue), and the movement of the hyoid bone. The studies had different protocols and outcomes, so it was not possible to establish standard procedures and normal or cut-off values for the diseases. The tools, methods, and techniques used in the reviewed studies varied widely, making it hard to assess them with common criteria. However, ultrasonography matched well with clinical evaluation of dysphagia and could have prognostic and rehabilitation value.27
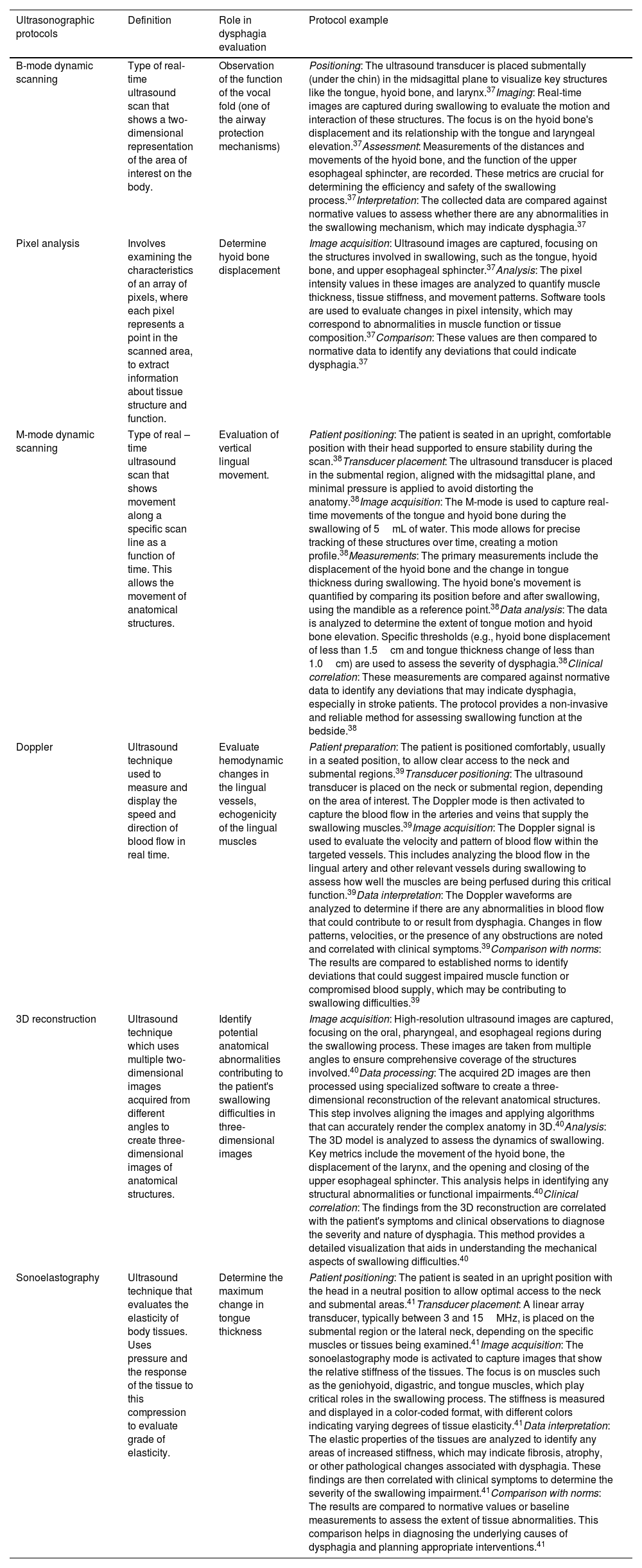
What evidence is there of ultrasonographic evaluation in dysphagia and what is it for?Some of the uses of ultrasonography in the evaluation of dysphagia are summarized in Table 2. Some of the protocols established in the literature for each of the US-modes of use are proposed.
Use of ultrasonographic protocols in dysphagia evaluation.
| Ultrasonographic protocols | Definition | Role in dysphagia evaluation | Protocol example |
|---|---|---|---|
| B-mode dynamic scanning | Type of real-time ultrasound scan that shows a two-dimensional representation of the area of interest on the body. | Observation of the function of the vocal fold (one of the airway protection mechanisms) | Positioning: The ultrasound transducer is placed submentally (under the chin) in the midsagittal plane to visualize key structures like the tongue, hyoid bone, and larynx.37Imaging: Real-time images are captured during swallowing to evaluate the motion and interaction of these structures. The focus is on the hyoid bone's displacement and its relationship with the tongue and laryngeal elevation.37Assessment: Measurements of the distances and movements of the hyoid bone, and the function of the upper esophageal sphincter, are recorded. These metrics are crucial for determining the efficiency and safety of the swallowing process.37Interpretation: The collected data are compared against normative values to assess whether there are any abnormalities in the swallowing mechanism, which may indicate dysphagia.37 |
| Pixel analysis | Involves examining the characteristics of an array of pixels, where each pixel represents a point in the scanned area, to extract information about tissue structure and function. | Determine hyoid bone displacement | Image acquisition: Ultrasound images are captured, focusing on the structures involved in swallowing, such as the tongue, hyoid bone, and upper esophageal sphincter.37Analysis: The pixel intensity values in these images are analyzed to quantify muscle thickness, tissue stiffness, and movement patterns. Software tools are used to evaluate changes in pixel intensity, which may correspond to abnormalities in muscle function or tissue composition.37Comparison: These values are then compared to normative data to identify any deviations that could indicate dysphagia.37 |
| M-mode dynamic scanning | Type of real – time ultrasound scan that shows movement along a specific scan line as a function of time. This allows the movement of anatomical structures. | Evaluation of vertical lingual movement. | Patient positioning: The patient is seated in an upright, comfortable position with their head supported to ensure stability during the scan.38Transducer placement: The ultrasound transducer is placed in the submental region, aligned with the midsagittal plane, and minimal pressure is applied to avoid distorting the anatomy.38Image acquisition: The M-mode is used to capture real-time movements of the tongue and hyoid bone during the swallowing of 5mL of water. This mode allows for precise tracking of these structures over time, creating a motion profile.38Measurements: The primary measurements include the displacement of the hyoid bone and the change in tongue thickness during swallowing. The hyoid bone's movement is quantified by comparing its position before and after swallowing, using the mandible as a reference point.38Data analysis: The data is analyzed to determine the extent of tongue motion and hyoid bone elevation. Specific thresholds (e.g., hyoid bone displacement of less than 1.5cm and tongue thickness change of less than 1.0cm) are used to assess the severity of dysphagia.38Clinical correlation: These measurements are compared against normative data to identify any deviations that may indicate dysphagia, especially in stroke patients. The protocol provides a non-invasive and reliable method for assessing swallowing function at the bedside.38 |
| Doppler | Ultrasound technique used to measure and display the speed and direction of blood flow in real time. | Evaluate hemodynamic changes in the lingual vessels, echogenicity of the lingual muscles | Patient preparation: The patient is positioned comfortably, usually in a seated position, to allow clear access to the neck and submental regions.39Transducer positioning: The ultrasound transducer is placed on the neck or submental region, depending on the area of interest. The Doppler mode is then activated to capture the blood flow in the arteries and veins that supply the swallowing muscles.39Image acquisition: The Doppler signal is used to evaluate the velocity and pattern of blood flow within the targeted vessels. This includes analyzing the blood flow in the lingual artery and other relevant vessels during swallowing to assess how well the muscles are being perfused during this critical function.39Data interpretation: The Doppler waveforms are analyzed to determine if there are any abnormalities in blood flow that could contribute to or result from dysphagia. Changes in flow patterns, velocities, or the presence of any obstructions are noted and correlated with clinical symptoms.39Comparison with norms: The results are compared to established norms to identify deviations that could suggest impaired muscle function or compromised blood supply, which may be contributing to swallowing difficulties.39 |
| 3D reconstruction | Ultrasound technique which uses multiple two-dimensional images acquired from different angles to create three-dimensional images of anatomical structures. | Identify potential anatomical abnormalities contributing to the patient's swallowing difficulties in three-dimensional images | Image acquisition: High-resolution ultrasound images are captured, focusing on the oral, pharyngeal, and esophageal regions during the swallowing process. These images are taken from multiple angles to ensure comprehensive coverage of the structures involved.40Data processing: The acquired 2D images are then processed using specialized software to create a three-dimensional reconstruction of the relevant anatomical structures. This step involves aligning the images and applying algorithms that can accurately render the complex anatomy in 3D.40Analysis: The 3D model is analyzed to assess the dynamics of swallowing. Key metrics include the movement of the hyoid bone, the displacement of the larynx, and the opening and closing of the upper esophageal sphincter. This analysis helps in identifying any structural abnormalities or functional impairments.40Clinical correlation: The findings from the 3D reconstruction are correlated with the patient's symptoms and clinical observations to diagnose the severity and nature of dysphagia. This method provides a detailed visualization that aids in understanding the mechanical aspects of swallowing difficulties.40 |
| Sonoelastography | Ultrasound technique that evaluates the elasticity of body tissues. Uses pressure and the response of the tissue to this compression to evaluate grade of elasticity. | Determine the maximum change in tongue thickness | Patient positioning: The patient is seated in an upright position with the head in a neutral position to allow optimal access to the neck and submental areas.41Transducer placement: A linear array transducer, typically between 3 and 15MHz, is placed on the submental region or the lateral neck, depending on the specific muscles or tissues being examined.41Image acquisition: The sonoelastography mode is activated to capture images that show the relative stiffness of the tissues. The focus is on muscles such as the geniohyoid, digastric, and tongue muscles, which play critical roles in the swallowing process. The stiffness is measured and displayed in a color-coded format, with different colors indicating varying degrees of tissue elasticity.41Data interpretation: The elastic properties of the tissues are analyzed to identify any areas of increased stiffness, which may indicate fibrosis, atrophy, or other pathological changes associated with dysphagia. These findings are then correlated with clinical symptoms to determine the severity of the swallowing impairment.41Comparison with norms: The results are compared to normative values or baseline measurements to assess the extent of tissue abnormalities. This comparison helps in diagnosing the underlying causes of dysphagia and planning appropriate interventions.41 |
It is prognostic regarding predicting whether a patient may be aspirating or not, but not confirmatory. It is projected as a screening evaluation to refer to more complex methods, as it is non-invasive and low-risk, paying special attention to hyoid movement, lingual thickness, and suprahyoid muscle size.21
Is diagnostic?It is projected as an evaluation of function and as a complement to video fluoroscopy and FEES.21 There is a difficulty with FEES, as it is not accessible to the entire community and not available in all healthcare institutions, in addition to concerns about radiation exposure, making it more oriented towards diagnosis.21 This method serves as a quantitative tool for assessing swallowing muscles, although not all suprahyoid muscles, by quantifying muscle thickness and degrees of contraction in real time.21 It allows for characterizations such as laryngeal elevation between the hyoid and thyroid, a process that varies according to the underlying pathology causing dysphagia.21 However, the sign of laryngeal elevation is not entirely reliable for determining aspirations or penetrations. When related to volume and viscosity, higher volumes and viscosity require greater ranges of movement by the hyolaryngeal complex, but this relationship is inversely related to age. This parameter, observable in ultrasound, is not only a diagnostic criterion but also allows for measuring treatment progress.21
Guidelines are provided to measure distances at rest and during movement of the hyolaryngeal complex for subsequent comparisons. Hyolaryngeal movement in ultrasound is considered because it is associated with the passage of food through the pharyngeal area, the opening of the upper esophageal sphincter (UES), and airway protection.22 For protocol standardization, measurements of distances, means, and percentages are considered; frequencies between 7 and 10Hz are recommended.22 The possibility of observing bolus passage through the airway during aspiration is enhanced by the acquisition of sequential images, starting from the B-mode image. This serves as a differential diagnostic parameter of dysphagia according to movement in centimeters, correlated with aspirations. However, it is important to note that penetration and aspiration are not the same, with aspiration carrying greater risk in dysphagia. To modulate contact between the transducer and the skin of the area to be evaluated for laryngeal tilting, it is suggested to place a water balloon.22
Does it allow intervention?Ultrasound allows for good resolution of the oropharyngeal musculature; however, studies primarily focus on describing tongue movement, hyolaryngeal movement, and cricopharyngeal function. Feedback therapy has shown positive therapeutic effects on skill acquisition, as reported in a patient with partial glossectomy. Intervention can also be guided for neurogenic swallowing disorders, such as the injection of botulinum toxin. Establishing mandibular reference points is essential for evaluating hyoid displacement during swallowing, considering the evaluation of tongue movement, laryngeal elevation, and suprahyoid muscles.22 Depending on the position of the translator, different components can be observed as shown in Table 3.
Correlation between transducer position and swallowing component observation.
| Transducer position or maneuvering | Component evaluated |
|---|---|
| The transducer placed in the midsagittal plane in the submental area | Mandible and hyoid bones. Those appear as two hyperechoic areas with acoustic shadows |
| Make a velar sound | Velum |
| Bisected the angle formed by the acoustic shadows of the hyoid and mandible | Tongue thickness |
| Two-axis coordinate system: the position of the hyoid bone in relation to the mandible in each frame is represented as coordinate pairs | Hyoid bone displacement. The distance between two coordinates before and during swallowing denote the hyoid bone displacement |
| The transducer is placed anterior to the thyroid cartilage | Ultrasonographic assessment of vocal cords |
| The transducer is placed transversely at the left of the trachea at the cricoid cartilage level | Ultrasonography assessment of the cricopharyngeal muscle |
The literature on critically ill patients is notably limited, with most studies primarily focusing on evaluating non-critical aspects of dysphagia. However, key principles from these studies can be adapted for application to critically ill patients. Although these methodologies and findings often center on less severe cases, they provide a foundational understanding that can be expanded for its use in more acute clinical scenarios.
In critically ill patients, airway ultrasound has proven to be valuable not only for airway management, but also for the diagnosis of dysphagia. Ultrasonography allows accurate anatomical assessment and helps to identify post-extubation complications and other respiratory problems, making it an essential tool.28
The utilization of ultrasound to assess oropharyngeal musculature, allows real-time visualization of muscle movements involved in swallowing. Recent studies have explored its use as a complementary tool in dysphagia evaluation, particularly in recently extubated patients. These studies emphasize the importance of ultrasound in early dysphagia detection, which could lead to timely intervention and improved clinical outcomes.29 Reviews of various post-extubation dysphagia interventions suggest that while ultrasound is not the sole tool used, its ability to assess both muscle function and air dynamics in the upper airway makes it a valuable asset in guiding dysphagia management. However, there is a recognized need for more high-quality research to validate its use in standardized protocols.30
For instance, one study assessed the utility of bedside ultrasound in detecting dysphagia in 19 patients with acute stroke.31 The study compared ultrasound measurements with the GUSS and FOIS scales, focusing on the distance between the hyoid bone and the larynx, which is typically reduced in dysphagia patients.31 A significant correlation was found between the hyoid-larynx distance and FOIS scores (P=0.011 and P=0.005, respectively) as well as GUSS scores (P=0.008 and P=0.004, respectively).31 The authors concluded that ultrasonography is a valuable tool for non-instrumental bedside evaluation of dysphagia in acute stroke patients, although its potential for intervention was not assessed, particularly in the context of critically ill patients with decreased levels of consciousness.31
Ultrasound has also been shown to be a useful tool in the assessment of swallowing function in post-stroke patients, indicating its potential in critical care settings. It has been shown to be effective in assessing oropharyngeal muscles and tongue and laryngeal movement, although further studies are needed to establish a universal consensus on examination protocols.5,32
Significant variations in examination techniques and methodologies underscore the need to establish a clinical diagnostic value for ultrasound in dysphagia. Current studies primarily focus on the tongue, hyolaryngeal movement, and the upper esophageal sphincter (UES), with limited attention given to detailed exploration techniques and pediatric dysphagia. The complexities in image analysis and measurement parameters, as well as the challenges in observing real-time aspiration, suggest that while structural observations are reliable, they lack comprehensive information on the progression of the food bolus during swallowing. Recommendations include integrating ultrasound parameters, conducting thorough evaluations of lingual muscles, pursuing prospective research to establish diagnostic value, and focusing on structural integrity, muscle contractile time, reference values, and echogenicity, particularly in diseased populations.5
Another study excluded videofluoroscopy to avoid radiation exposure; however, the use of a syringe to administer water may disrupt the natural swallowing process.22 The diagnostic criteria for penetration and aspiration remain unclear, and the margin of error for ultrasound increases when applied to larger populations.22 False negatives necessitated the use of an additional ultrasound technique to observe air bubbles mixed with water droplets, indicating that hyolaryngeal movement alone is insufficient for diagnosing pharyngeal dysphagia.22 Recommendations suggest using ultrasound to measure hyolaryngeal movement at the bedside, performing measurements both at rest and during swallowing for comparative analysis, and considering ultrasound as a non-invasive alternative for evaluating dysphagia in children with cerebral palsy.22
There is a poor correlation between hyolaryngeal displacement data obtained from videofluoroscopy and ultrasound, along with inadequate data acquisition. For reliable assessment, it is essential to have homogeneous sample data and to consider speech pathology criteria as well as differential diagnoses. The study encountered limitations due to the small size of the ultrasound device and the subjective inclusion of patients with hyolaryngeal involvement, leading to distortion of statistical data. Recommendations suggest that the clinical use of the Clarius device for swallowing evaluation is premature, advocating for increased data acquisition and emphasizing the need for improved scanning techniques and image selection to achieve reliable assessments.33
DiscussionThe role of ultrasonography in the evaluation of dysphagia in critically ill patients presents significant promise but also reveals several limitations and areas needing further research and standardization. This narrative review highlighted various facets of ultrasound application, revealing both its strengths and weaknesses in clinical practice.6
Ultrasound in the evaluation of dysphagia is primarily used for assessing tongue movement, hyolaryngeal movement, and upper esophageal sphincter (UES) function. However, significant variations in examination techniques and methodologies exist, necessitating the establishment of a clear clinical diagnostic value for ultrasound in dysphagia.27 The studies reviewed often focus on structural observations but lack comprehensive data on the progression of the food bolus during swallowing, which is crucial for accurate dysphagia diagnosis.
The Gugging Swallowing Screen (GUSS) stands out as the protocol with the most evidence, especially in patients with stroke or acquired neurological injuries. Its high sensitivity and specificity make it a valuable tool, though it primarily provides structural observations without detailed information on bolus progression.14,16 The volume viscosity swallow test is another essential tool, particularly in ICU settings, due to its rapid and easy application.34 However, limitations in using methylene blue in the EVANS test due to toxicity risks highlight the need for safer alternatives like vegetable blue dye.13
In recent years, the cough reflex test has fallen out of favor and has largely been replaced by the first part of the GUSS, which evaluates both voluntary and reflexive cough. The water swallow test is now the preferred screening tool and can also be applied to neurological patients, incorporating the YALE PROTOCOL, which includes the Water Test. The GUSS protocol is particularly notable for its high sensitivity and specificity compared to videofluoroscopy and FEES, making it relevant in assessing various types of dysphagia.6
Despite its utility, the EVANS test has several limitations, including its design for non-ventilated tracheostomized (TQT) patients who can tolerate occlusion and deflated cuff, alertness, and compliance with commands. The high incidence of false negatives in this test necessitates supplementary VDF or FEES. The SHEBA PROTOCOL was developed to address these limitations for TQT and ventilated patients, integrating the blue dye test with a sequence similar to VVST. Cervical auscultation remains a valuable tool, though it heavily depends on the auditory training of the speech therapist.35
When considering the weaknesses in diagnostic parameters of FEES and VFSS, it is important to weigh the pros and cons of endoscopy and fluoroscopy. FEES may be preferred for evaluating patients with dysphonia, as it allows direct observation of laryngeal function for adduction and airway protection. However, FEES cannot observe the complete oral phase of swallowing and may miss aspiration events during endoscopic “blanching” that occurs during pharyngeal constriction. These limitations have led some experts to question the accuracy of FEES for diagnosing oropharyngeal dysphagia.17
On the other hand, VFSS has deficiencies related to quality and analysis, such as patient cooperation, video quality, imaging personnel's technical expertise, and examination methodology. During VFSS, the usual conditions of feeding are not replicated as a radio-opaque contrast with specific texture and volume is used.18 Despite these issues, VFSS remains the gold standard due to its ability to evaluate all stages of swallowing, including gastric emptying and laryngeal ascent.25,26
Ultrasound's utility extends to non-invasive screening, making it a preferred choice in scenarios where traditional methods like FEES and VFSS might pose accessibility or radiation risks. Yet, the diagnostic criteria for penetration and aspiration remain unclear in some studies, with a significant margin of error when extrapolated to larger populations. This issue underscores the need for supplementary techniques to enhance diagnostic accuracy.21
In children with cerebral palsy, ultrasound shows potential as a non-invasive alternative for evaluating dysphagia. However, the method of providing water via a syringe in some studies may alter the normal swallowing process, potentially compromising the integrity of the biomechanical assessment. This factor, along with the need for clear diagnostic criteria for penetration and aspiration, points to areas requiring further refinement.36
The validity and reliability of pocket-sized ultrasound systems in dysphagia assessment are also under scrutiny. Studies indicate a poor correlation between hyolaryngeal displacement data from videofluoroscopy and ultrasound, insufficient data acquisition, and subjective inclusion criteria leading to statistical distortions. These findings suggest that while portable ultrasound devices offer convenience, their clinical use for swallowing evaluation is not yet recommended without further data acquisition and improved scanning techniques.5
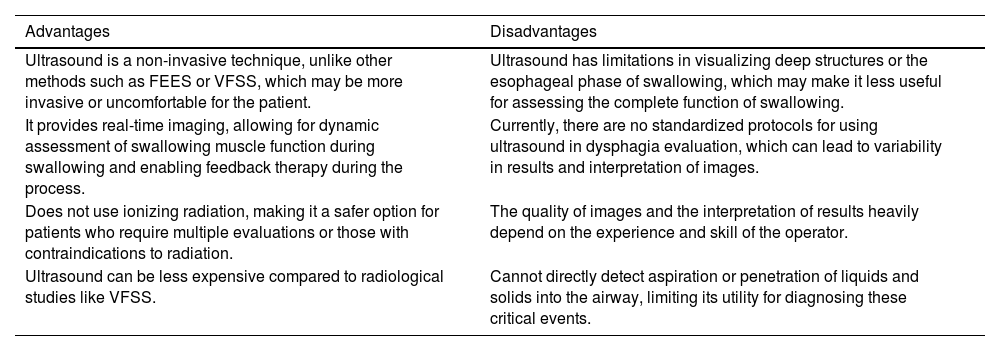
Ultrasound allows for good resolution of the oropharyngeal musculature and has shown positive therapeutic effects in feedback therapy, particularly in patients with partial glossectomy. It also serves as a guide for interventions in neurogenic swallowing disorders, such as botulinum toxin injections. Establishing mandibular reference points is crucial for evaluating hyoid displacement during swallowing, providing insights into tongue movement, laryngeal elevation, and suprahyoid muscle function.22 The advantages and disadvantages of using US for the evaluation and diagnosis of dysphagia in critically ill patients compared to other tests are presented in Table 4.
Advantages and disadvantages of using ultrasound for dysphagia diagnosis compared to other tests.
| Advantages | Disadvantages |
|---|---|
| Ultrasound is a non-invasive technique, unlike other methods such as FEES or VFSS, which may be more invasive or uncomfortable for the patient. | Ultrasound has limitations in visualizing deep structures or the esophageal phase of swallowing, which may make it less useful for assessing the complete function of swallowing. |
| It provides real-time imaging, allowing for dynamic assessment of swallowing muscle function during swallowing and enabling feedback therapy during the process. | Currently, there are no standardized protocols for using ultrasound in dysphagia evaluation, which can lead to variability in results and interpretation of images. |
| Does not use ionizing radiation, making it a safer option for patients who require multiple evaluations or those with contraindications to radiation. | The quality of images and the interpretation of results heavily depend on the experience and skill of the operator. |
| Ultrasound can be less expensive compared to radiological studies like VFSS. | Cannot directly detect aspiration or penetration of liquids and solids into the airway, limiting its utility for diagnosing these critical events. |
In summary, while ultrasonography presents a valuable tool in the assessment and intervention of dysphagia, significant gaps in standardization, diagnostic criteria, and comprehensive evaluation methods need to be addressed. Future research should focus on integrating useful ultrasound parameters, conducting comprehensive evaluations, and establishing standardized protocols to enhance the diagnostic and therapeutic utility of ultrasonography in dysphagia.
ConclusionUltrasonography shows significant potential in evaluating and managing dysphagia in critically ill patients due to its non-invasive nature and real-time feedback capabilities. However, variations in techniques and the need for standardized diagnostic criteria present challenges. While protocols like the Gugging Swallowing Screen (GUSS) and the volume viscosity swallow test are valuable, limitations in tests like the EVANS test necessitate supplementary evaluations. Future research should focus on standardizing ultrasound parameters and refining diagnostic criteria to enhance its reliability and accuracy. Despite these challenges, ultrasonography remains a promising tool for dysphagia assessment and intervention.
FundingNo funding was received from any organization or entity for the research and preparation of this work. All contributions to this manuscript were made independently by the authors, ensuring the integrity and impartiality of the study.
Conflicts of interestThe authors declare that there are no conflicts of interest regarding the publication of this manuscript.