Autosomal Dominant Polycystic Kidney Disease (ADPKD) is one of the most prevalent inherited disorders in the world. It's a systemic disease with preferential renal involvement. However, many other organs can be affected, causing important morbidity. Some cases of male infertility are described in literature. That seems to be related to abnormalities in spermatozoa tail microtubules, atonicity and dilatation of seminal vesicle or distal seminal tract cysts and anomalies associated with chronic renal failure. This article presents a patient who combines the two major mechanisms described in literature for male infertility in ADRKD: immotile spermatozoa and megavesicles. This coincidence supports the idea of a common etiological factor to kidney and infertility anomalies.
© 2014 Associação Portuguesa de Urologia. Published by Elsevier España, S.L.U. All rights reserved.
A Doença Renal Poliquística Autossómica Dominante (DRPAD) é uma das doenças hereditárias mais prevalentes no mundo. Trata-se de uma doença sistémica com envolvimento renal preferencial. No entanto, outros orgãos podem ser afetados, causando morbilidade importante. Alguns casos de infertilidade são descritos na literatura e parecem estar relacionados com alterações nos microtúbulos da cauda do espermatozóide, com atonia e dilatação das vesículas seminais ou quistos do trato seminal distal bem como anomalias relacionadas com doença renal crónica. Este artigo apresenta um doente que combina dois mecanismos major de infertilidade na DRPAD: espermatozóides imóveis e megavesículas. Esta coincidência aponta para a ideia de uma etiologia comum entre as anomalias renais e da infertilidade.
© 2014 Associação Portuguesa de Urologia. Publicado por Elsevier España, S.L.U. Todos os direitos reservados.
ADPKD is one of the most prevalent inherited disorders, affecting 1 in each 800-1000 live births.1 It's responsible for end-stage renal disease (ESRD) in 4.4% of patients on renal replacement therapy, worldwide.2
ADPKD is a systemic disease with preferential renal involvement. However, many other organs can be affected, causing important morbidity. Although men with ADPKD are usually fertile, some cases of male infertility are described in literature. Being a common genetic disease, it's important to be conscious of its reproductive implications. Literature about male infertility in ADPKD is supported almost exclusively by case reports and small studies. However, several anomalies were found in men with ADPKD such as asthenozoospermia, necrospermia, hipospermia, oligospermia or azoospermia, and dilatation of seminal vesicle (SV) or distal seminal tract cysts.
The objective of the present article is to describe an infertile man with ADPKD who comprises some anomalies suggestive of corresponding to the infertility etiopathogenic mechanisms described in literature. Starting from a revision of the available literature it is intended to understand if there is a common aetiology mechanism between renal cysts and anomalies related with infertility.
Case reportA 27years old male with ADPKD was sent to Male Infertility Outpatient Clinic for primary infertility. The couple complained of unsuccessful conception after 2year of unprotected sexual intercourse and brought a semen analysis indicating hipospermia and oligoasthenozoospermia. As risk factors for infertility the patient had a previous surgery to bilateral hydrocele and inguinal hernia in infancy and possible occupational exposure (industrial worker). Family history was negative for infertility; his mother also had ADPKD.
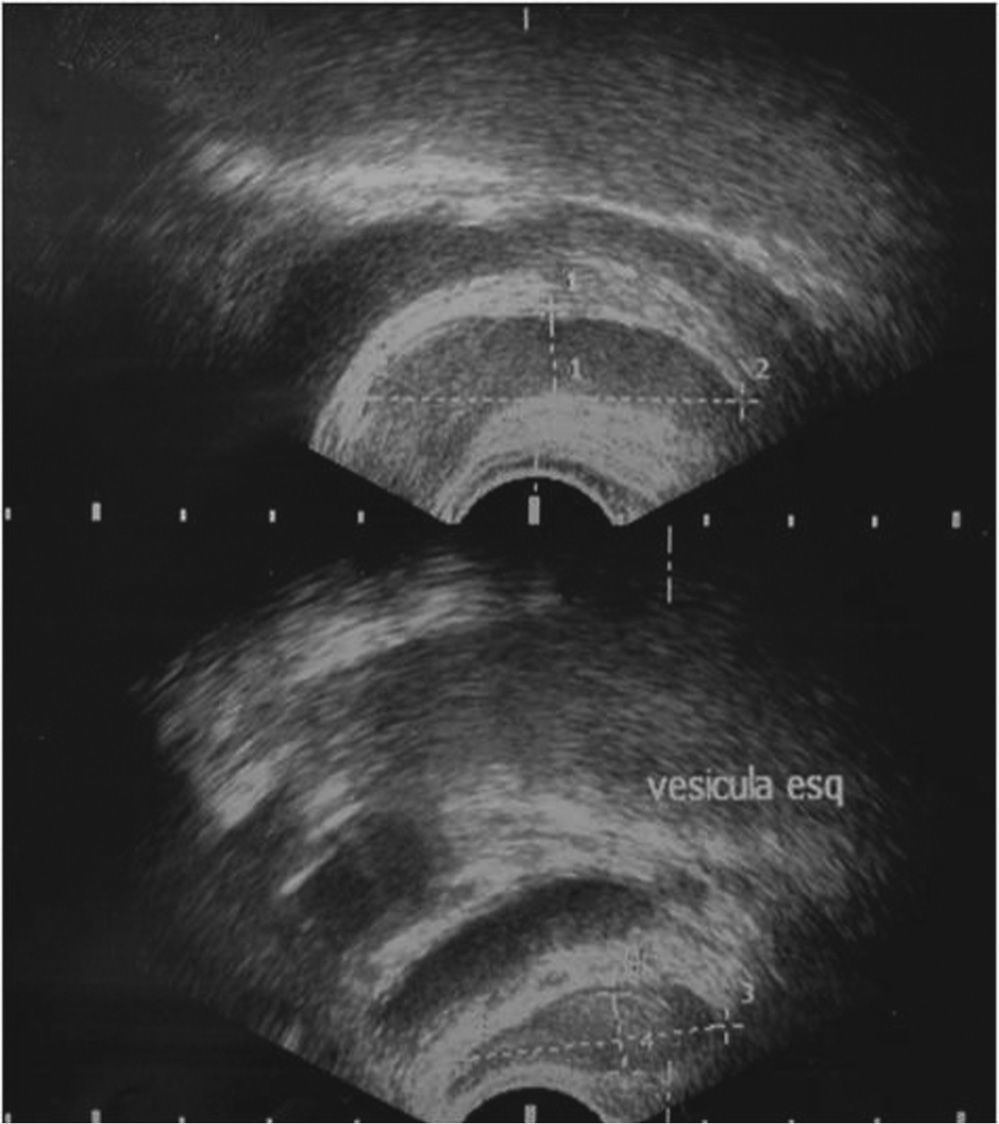
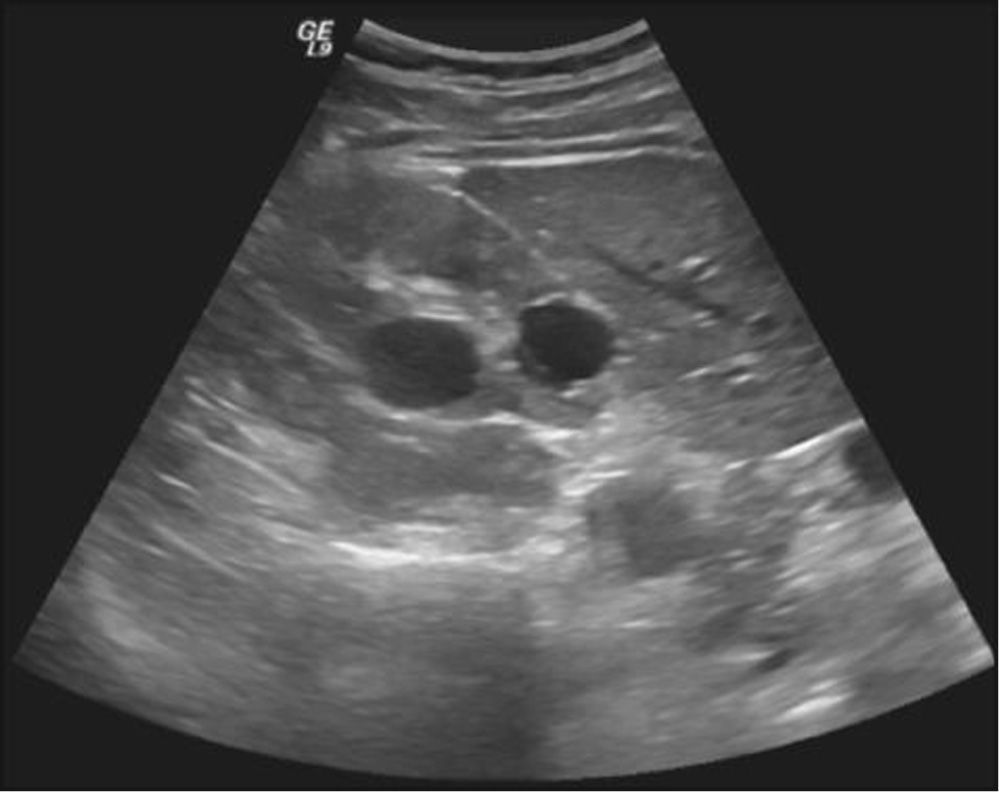
Physical examination revealed no alterations. Blood analysis showed hormonal, lipid, glycaemic e renal profiles within normal range. Sperm analysis, repeated 3months after the first one, revealed hipospermia (sperm volume under 1.5mL) and criptozoospermia (scarce sperm concentration after sample centrifugation). Sperm culture was negative. Sperm analysis showed normal lead, sulphur and chromium concentrations. Ultrasound study detected the presence of multiple renal cysts bilaterally, the larger with 20-30mm; normal size and density testes, with symmetric and preserved vascularization; presence of two cysts of 2 and 3mm at left epididymis; subclinical varicocele on left; normal prostate with a volume of 22mL; dilated seminal vesicle: left with 9.4 × 44.0 × 31.9mm and right with 10.0 × 43.2 × 32.1mm, without cysts (figs. 1-2).
Once transretal ultrasound was performed, it was decided to proceed to aspirative puncture of seminal vesicles, expecting to collect a better sperm sample. It revealed the presence of viable immotile spermatozoa, but unfortunately cryopreservation was not possible due to excess of mucus in the sample.
At the present time the couple is waiting for genetic counsel consultation. The patient will be proposed for percutaneous epididymal sperm aspiration to proceed to in vitro fertilization.
Discussion and conclusionsADPKD is a systemic genetic disease especially common around the world. It has an autosomal dominant inherited pattern with 100% penetrance; therefore the probability of transmission to descendants is 50%. There are two types of ADPKD according to the mutated gene. Type 1 ADPKD, responsible for about 85% of cases, is caused by mutations on the polycystic kidney disease gene (PKD) 1, mapped to 16p13.3. Type 2 ADPKD, responsible for about 15% of cases, is caused by mutations on the PKD 2, mapped to 4q13-23. There is some suspicion about the existence of a third gene, PKD3, but it's still unmapped.3
These genes encode transmembrane proteins named polycystin (PC) 1 and 2, which have been the focus of multiple studies lately. It has been demonstrated that PC1 and PC2 are located at renal tubular cells apical cilia, where they translate extracellular stimuli. PC1 works as a mechanoreceptor of the luminal fluid flow. When stimulated, its structure changes and turns on PC2. PC2 is a non-selective cationic channel permeable to calcium. PC1 e PC2 work together, increasing the intracellular calcium concentration and leading to a normal development of the kidney.2
ADPKD is characterized by the presence of multiple renal cysts bilaterally. It's though a systemic disease, with several extra-renal manifestations, which can have cystic or non-cystic phenotypes. Cysts can develop in other organs as liver, pancreas, spleen, arachnoid mater, epididymis, prostate, seminal vesicle and ejaculatory ducts as in ovary and uterus. Non-cystic manifestations include vascular aneurysms, cardiac valvular anomalies, colic diverticula and abdominal wall hernia.
Bellet et al estimated that about 5% of ADPKD men are infertile.4 Fang et al concluded that ADPKD prevalence in non-fertile men was higher than expected.5 In western countries, general prevalence of infertile couple is 15% and infertile men is 7%.6 There are no randomized studies that confirm this relation. It is possible that infertility is undiagnosed on ADPKD patients, or ADPKD is undiagnosed on some infertile men.
Nevertheless, according to published literature, there are several etiologic mechanisms that justify anomalies found in patients with ADPKD:
- (1)
Spermatozoa tail anomalies
Okada et al showed that the architecture of spermatozoa tail with two central microtubules surrounded by nine peripheral microtubules (axoneme) is crucial for spermatozoa motility and fertilization. By studying non-fertile men with immotile 9+0, instead of normal 9+2 microtubules spermatozoa, they found out that they were all carriers of ADPKD.7 This anomaly justifies asthenozoospermia observed in ADPKD patients as well as necrospermia, for stasis of immotile spermatozoa.
Polycystins, proteins changed in ADPKD, are located on tubular renal cell cilia. Cilia share its ultrastructure with spermatozoa flagellum. Watnick et al verified the presence of PC2 at the distal tip of the spermatozoa flagellum in Drosophila melanogaster. They also concluded that a targeted mutation on the gene caused male sterility.8
These facts point out the possibility that anomalies in human spermatozoa tail may be also originated from the same mutation on PDK, such as the one that happens in the kidney.
- (2)
Seminal vesicle anomalies
In ADPKD population, SV cysts represent a common extra-renal manifestation with a prevalence of 39%-60%.9 In general population, SV cysts prevalence rounds only 5%.6 Even so, these cysts rarely relate to infertility. There are some cases described in literature in which the presence of distal seminal tract cysts caused obstruction to the passage of ejaculated, therefore leading to hipospermia and oligospermia or azoospermia.10
However, the mechanism that most often causes these anomalies seems to be functional and not obstructive. Studies with percutaneous vesiculography in ADPKD patients showed that apparent SV cysts on ultra-sound images were really dilated and tortuous SV, without evidence of obstruction.11 These were called megavesicles. Microscopic observations of megavesicles aspirate confirmed the presence of sperm stuck within, but after transurethral resection of ejaculatory ducts (TURED), sperm analysis didn't improve significantly.12 These data suggest that the subjacent issue is probably a functional inability of propulsion of SV contents, instead of a truly obstruction.
Studies in corpses with megavesicles showed that vesicle and ejaculatory ducts muscle wall was thinner or even absent in these patients. This finding corroborates the idea of dyskinesia and atonicity of SV as the cause of its dilation.11
SV atonicity justifies the presence of azoospermia/oligospermia and hipospermia, once sperm isn't totally pushed to urethra, as well as necrospermia, for stases and death of spermatozoa that remains shucked in VS.4
- (3)
Anomalies associated with Chronic Renal Failure (CRF)
CRF damages reproductive male system at all levels of hypothalamus-pituitary-gonadal axis. This damage is not reverted by dialyses and it is only partially reverted by renal transplant. Xu et al found a fertility index in CRF patients of 0.23, in comparison to 13.2 on healthy control group.13
Uraemia that courses with CRF has a cytotoxic effect on testicular cells, compromising spermatogenesis. It also affects hormonal regulation of Leydig and Sertoli cells, compromising steroidogenesis. Gonadotropin levels are elevated in CFR, not because higher production occurs at pituitary level, which is indeed diminished, but because of a significant reduction of LH renal clearance.16
Together with hormonal, vascular and autonomous anomalies contribute to erectile dysfunction and minor libido, which affects about 50% of CRF patients.4
CRF and uraemia take place, sooner or later, in ADPKD, making worse other causes of infertility, already present on these patients.
The patient presented above had normal renal and hormonal function, excluding CRF as the infertility cause. His ultrasound revealed a subclinical varicocele. According to current literature, it could justify oligoasthenozoospermia but not hipospermia or megavesicles. No evidence formally indicates, anyway, that treatment of subclinical varicocele improves fertility. Sperm analysis showed lead, sulphur and chromium concentration below toxic levels, excluding also occupational exposure as the infertility cause.
Curiously, our patient seems to combine precisely the two major mechanisms described in literature for male infertility in ADPKD:
- –
He had hipospermia and oligospermia, as well as megavesicles, features that course with SV dyskinesia and atonicity.
- –
He had viable, though immotile spermatozoa, which feeds in the spermatozoa tail disorder.
- –
This coincidence makes us inevitably think about a common etiological factor to kidney and infertility anomalies. Studies need still to be done, to confirm if polycystins are expressed on spermatozoa tail and seminal vesicle tissues, and how they relate to verified changes. Nonetheless, patients with ADPKD should be investigated with sperm analysis and transrectal ultra-sound, so infertility can be detected, and treatment can be provided on time.
Conflicts of interestThe authors have no conflicts of interest to declare.