To evaluate the relationship between mean apparent diffusion coefficient (ADC) and post-surgical Gleason scores. To determine the diagnostic accuracy of multiparametric magnetic resonance imaging (mp-MRI) on a 1.5T magnet in distinguishing low, intermediate and high-grade prostate tumors.
Material and methodsThis is a retrospective institutional-review-board-approved, single-center study including 30 patients (median age, 60 years) who underwent mp-MRI before prostatectomy for prostate cancer. Using histological reports for guidance, the tumors were localized in ADC maps, and mean ADCs were measured and examined for correlation with Gleason scores. 2 patients had 2 measurable foci, so a total of 32 tumors were studied. The diagnostic accuracy of the mean ADC was assessed by using the area under the receiver operating characteristic curve (ROC).
ResultsIn the differentiation of tumors with a Gleason score of 6 from those with a Gleason score of at least 7, mean ADC yielded an AUC of 0.76 (95% confidence interval: 0.59, 0.93). In the differentiation of tumors with Gleason scores of 6 or 7 from those with a Gleason score of at least 8, mean ADC yielded an AUC of 0.94 (95% confidence interval: 0.86, 1.00).
ConclusionMean ADC values may allow a correct assessment of the patient risk using a 1.5T magnet without ERC.
Avaliar a relação entre os valores de coeficiente aparente de difusão (ADC) e os scores de Gleason (SG) pós-cirúrgicos e determinar a acuidade diagnóstica da ressonância magnética multiparamétrica (RM-Mp) 1.5T sem sonda endorretal na distinção de carcinomas da próstata de baixo, intermédio e alto grau.
Material e métodosEstudo retrospetivo, científica e eticamente aprovado, incluindo 30 doentes (idade média: 60 anos) submetidos a RM-Mp pré-prostatectomia. Utilizando os relatórios histológicos como guia, os tumores foram localizados nos mapas de ADC, com vista a quantificar os coeficientes. Dois doentes apresentaram 2 focos mensuráveis, pelo que foram estudados 32 tumores. A relação entre os valores de ADC e o SG foi analisada através do coeficiente de correlação de Spearman. Para avaliar a acuidade diagnóstica dos valores ADC, foram obtidas receiver operating characteristic curves (curvas ROC).
ResultadosOs valores de ADC mostraram uma correlação negativa significativa com o SG. Na diferenciação de tumores com SG de 6 e SG≥7, obteve-se AUC de 0,76 (intervalo de confiança 95%: 0,59; 0,93). Na diferenciação de tumores com SG de 6 ou 7 e SG≥8, obteve-se AUC de 0,94 (intervalo de confiança 95%: 0,86; 1,00).
ConclusãoA medição dos valores de ADC num aparelho de 1,5T sem sonda endorretal é útil na estratificação de risco do cancro da próstata.
The use of multiparametric magnetic resonance imaging (mp-MRI) has significantly changed the diagnostic approach and management of prostate cancer. It combines the conventional sequences, T1 and T2 weighted imaging (WI), with at least two functional studies which may include diffusion weighted imaging (DWI), dynamic contrast-enhanced (DCE) and spectroscopy.1,2
Detection, staging, tumor aggressiveness assessment, and recurrence suspicion constitute its main indications. The adding of functional studies improved the accuracy of MRI and allowed the analysis of new parameters like tumor aggressiveness that inspires the use of mp-MRI in active surveillance. Both DWI and spectroscopy allow this assessment, the first by studying the effect of the increased cellular density on free water motion, and the second by analyzing changes on the concentration of some metabolites like choline, creatine and citrate.3–5
This functional imaging technique assesses the random movement of water molecules in different physical media. It is possible through the application of diffusion-sensitizing gradients with distinct strengths, known as b values and measured in seconds per square millimeter (s/mm2). It means that with a b value of 0s/mm2, there is no gradient and the signal intensity is based on T2 weighting. At high b values, such as 1000s/mm2, the water molecules within a highly cellular tissue retain their high signal and persist bright on DWI, as may occur within a tumor. In contrast, tissues containing water moving freely, like the bladder, will lose their signal.6–9
DWI allows a quantitative analysis through the calculus of the apparent diffusion coefficient (ADC), measured in square millimeters per second. This quantification is displayed parametrically in a gray-scale map, where areas of restricted diffusion appear with a darker shade of gray (lower ADC values) and tissues with freely moving water show lighter shades of gray (higher ADC values). Some studies have shown a significant negative correlation between the ADC values of prostate tumors and prostatectomy specimen Gleason scores. Moreover, ADC values appear to perform better than TRUS biopsy Gleason scores in the association with prostatectomy Gleason scores. ADC values may allow a risk stratification and correct guidance of low-risk patients toward active surveillance.6,10,11
This manuscript will focus on the role of DWI in evaluating tumor aggressiveness. The purpose of our study was to evaluate the relationship between mean ADCs and post-surgical Gleason scores, and to determine the diagnostic accuracy of mp-MRI on a 1.5T magnet without endorectal coil in distinguishing low, intermediate and high-risk prostate tumors.
Material and methodsThis is a retrospective institutional-review-board-approved, single-center study.
PatientsWithin a 2-year period (from January 2013 to December 2014), 198 patients underwent prostate mp-MRI for detection, staging and active surveillance purposes. Among these patients, 32 patients with biopsy-proven cancers underwent radical prostatectomy. Two patients were excluded from the study because of motion artifacts on MRI. The remaining 30 patients were included in the study (median age, 60 years, range 50–74).
MRI protocolMp-MRI studies were performed on a 1.5T body scanner (Magnetom Avanto; Siemens) with a 33mT/m maximum gradient capability using an eight channel pelvic phased array (PPA), without endorectal coil (ERC). Peristalsis was not suppressed. In order to avoid post-biopsy hemorrhage, mp-MRI was performed at least 6 weeks after biopsy.
The study of the pelvis included an axial turbo spin-eco T1WI imaging and an axial blade T2WI with fat saturation. With regard to the prostate gland study, we performed a set of axial, coronal and sagittal high-resolution T2WI (3mm; gap 0.6mm; FOV 20cm; matrix 310×320). An axial DWI with b values of 0, 50, 1000 and 1200s/mm2, and ADC maps were automatically generated by the imager software. DCE-MRI using an axial fat-saturated 3D Vibe T1W MR sequence after administration of gadoterate meglumine, with a dose of 0.2mmol/kg of body weight as a bolus injection, was also performed.
Imaging and histological analysisTwo radiologists with 3 years of experience in interpreting mp-MRI worked in consensus and reviewed all images on a workstation (Advantage, GE Healthcare). Using histological reports for guidance, the tumors were retrospectively localized in DWI and ADC maps, as bright and dark areas, respectively. Axial T2WI and DCE sequences were synchronized with the ones above for a better localization of the lesions and a clearer anatomic depiction. T1WI were also reviewed in order to exclude hemorrhage-related artifacts.
Six sextants were considered: left base, right base, left midgland, right midgland, left apex, and right apex. Each sextant was also divided into anterior, posterior, lateral, and medial sections. When possible, each sextant was further divided into central and peripheral gland. All these divisions were considered and reported in order to precisely localize the tumors and achieve a better association with the histologic reports. Only tumors originating in the peripheral zone were included in our study.
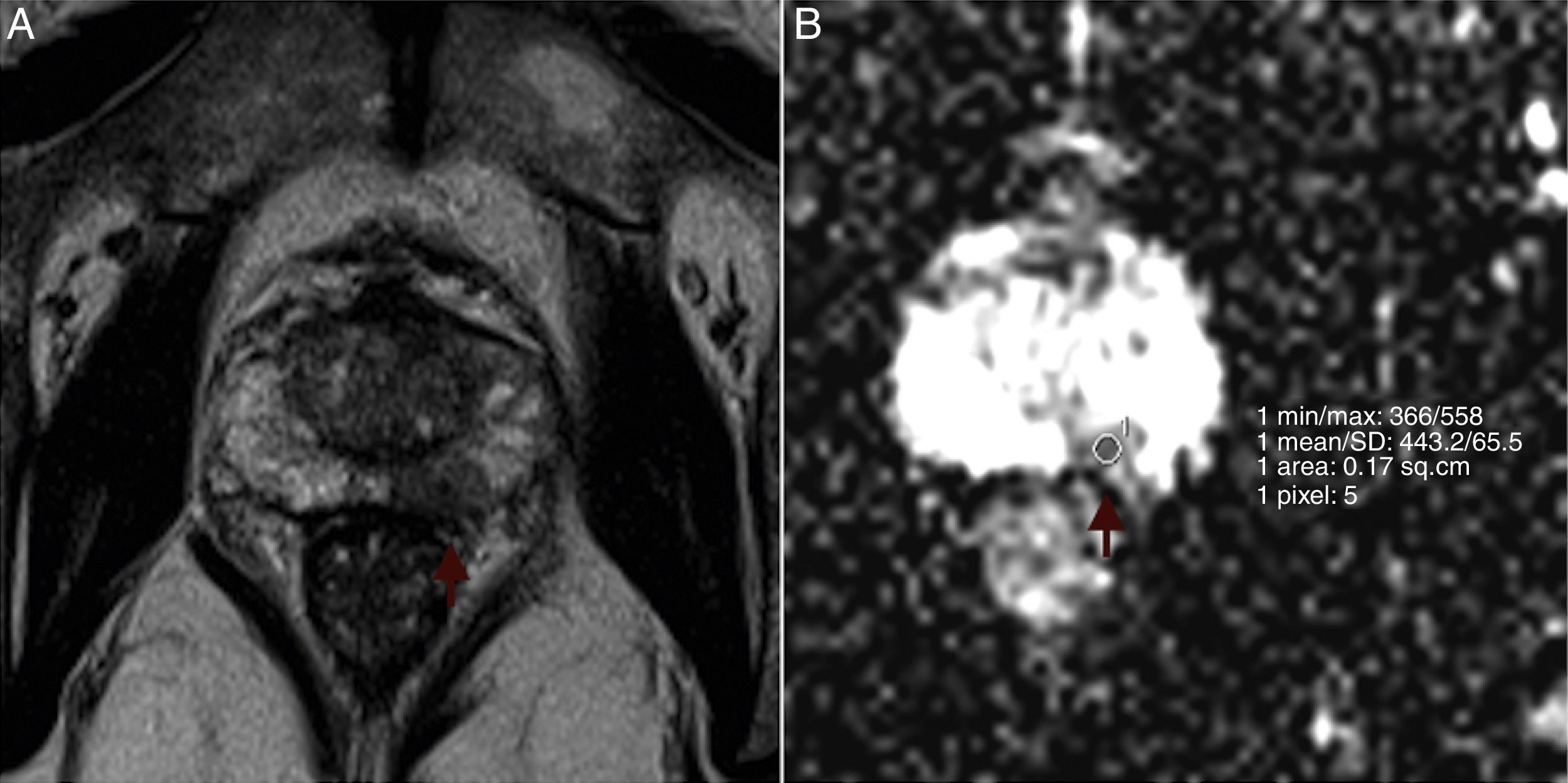
Mean ADCs were measured and examined for correlation with Gleason scores. The radiologist was blinded to the Gleason scores. All measurements were performed through the application of a single slice region of interest (ROI) within the tumor in the ADC map, trying to avoid tumor edges. ROIs had the same size for all tumors (27mm2). In multifocal tumors, the two largest foci were considered for ADC measurement. In large, heterogeneous tumors, ROIs were placed in the darkest area of the tumor. Due to limitations in spatial resolution, tumors smaller than 5mm in bigger axis were not studied. Fig. 1 shows an example of ROI placement. Among the 30 patients, 2 had multifocal pathology with volume enough to measure on the ADC map, so a total of 32 tumors were studied.
All prostatectomies were performed within 15 days of mp-MRI, and no treatment was implemented between them. Two pathologists with 5 years of experience reviewed the specimens and were blinded to the MRI results. The prostates were received fresh from the operating room, and both weight and dimensions were recorded. All specimens were then covered with two different color inks and fixed for 24h in 4% neutral buffered formalin. After fixation, the apex and base were removed from each specimen as thin shave margins, and the seminal vesicles were amputated, cut into two halves and processed in toto. Then midglands were sectioned at 5mm intervals perpendicular to the long axis of the gland. After that the cut specimens were dehydrated in graded alcohols, cleared in xylene, embedded in paraffin, and examined histologically as 5μm-thick whole-mount hematoxylin and eosin (H&E) stained sections. Each slice was examined for the presence of gross and/or microscopic lesions. Similarly to the MR analysis, six sextants were considered: left base, right base, left midgland, right midgland, left apex, and right apex. Each sextant was also divided into anterior, posterior, lateral, and medial sections. Each tumor was measured in three dimensions and a Gleason score was given. When several foci were found, Gleason scores were given separately. In order to better locate the tumor and improve correlation with MRI findings, the shortest transversal and longitudinal distances were measured between each lesion and the capsule and base and/or apex margins, respectively.
Statistical analysisTwo variables were considered: ADC values, a numeric variable, and Gleason scores, an ordinal variable. In relation to the Gleason scores, eight different grade groups were identified according to the primary and secondary features present. Gleason score was discretized into two new binary variables (score 6 vs score higher than 6; scores 6, 7 vs scores 8, 9). An exploratory analysis was carried out for all variables. Continuous variables were presented as mean or median, standard deviation (SD) or inter-quartile range (25th percentile–75th percentile), as required.
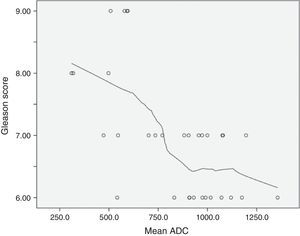
The relationship between ADCs and ordinal Gleason score groups was firstly analyzed by applying Spearman's correlation coefficient. Data was also displayed in a scatterplot. Moreover, in order to study the diagnostic accuracy of the mean ADC, logistic regression models were fitted to the data considering the two previous defined binary variables as the outcome. Predictive and discriminative abilities of the models were assessed by the Hosmer–Lemeshow goodness of fit test and by the area under the receiver operating characteristic curve (ROC), respectively. A model with good fit will have a lower observed Hosmer–Lemeshow chi-square statistic value and a non-significant p-value. A level of significance α=0.05 was considered. Statistical analyses were performed with software (SPSS, version 22.0.01).
Results30 patients were included in the study, and a total of 32 tumors were considered to ADC measurement. 12 (37.5%), 13 (40.7%), and 7 (21.4%) had Gleason scores of 6, 7, and more than or equal to 8, respectively.
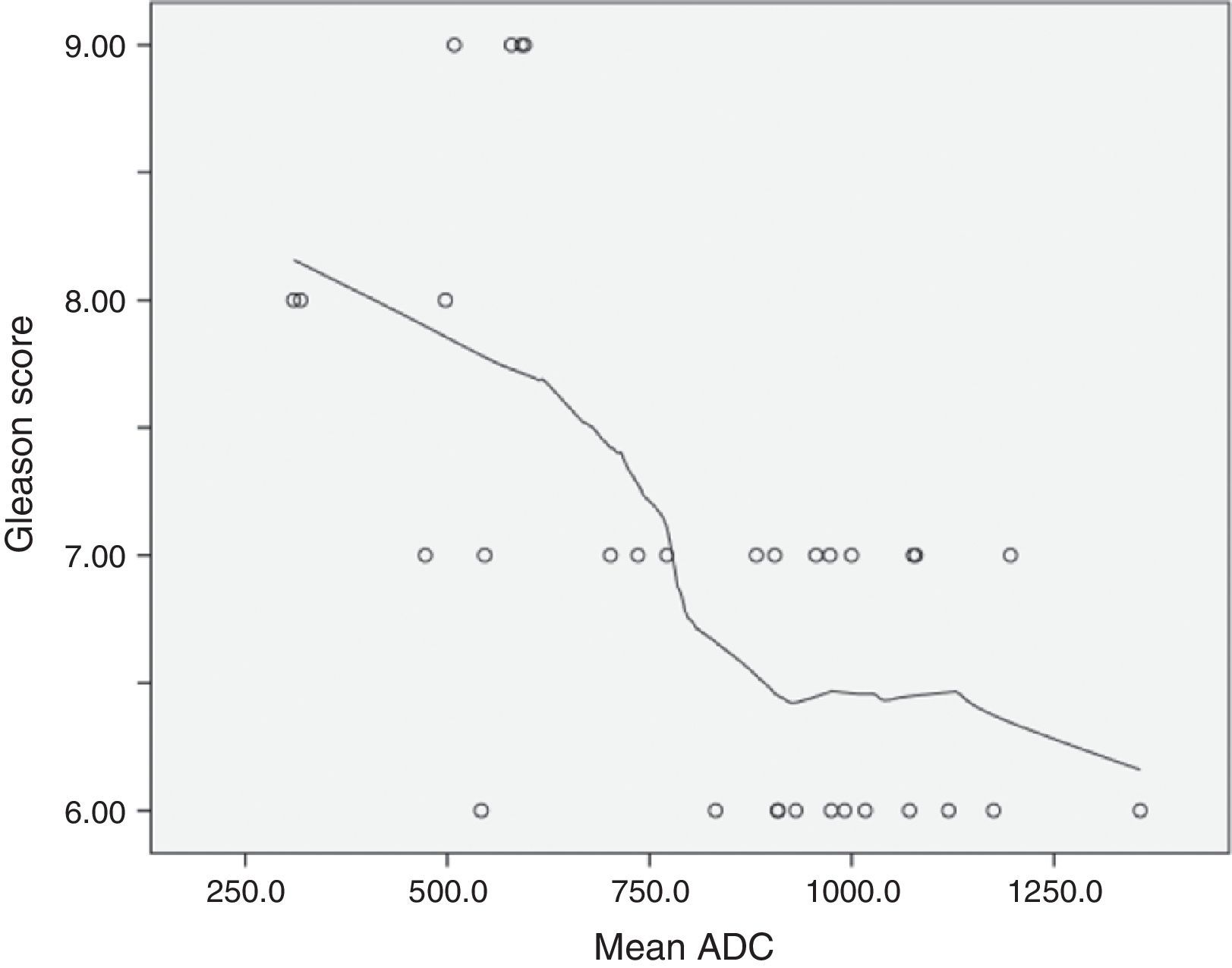
Mean ADC showed a significant negative correlation with Gleason ordinal scores. The Spearman p value for mean ADC was −0.594 (p<0.001). According to the scatterplot, some overlap is found between the ADCs measured in tumors with Gleason scores of 6 and 7 (Fig. 2).
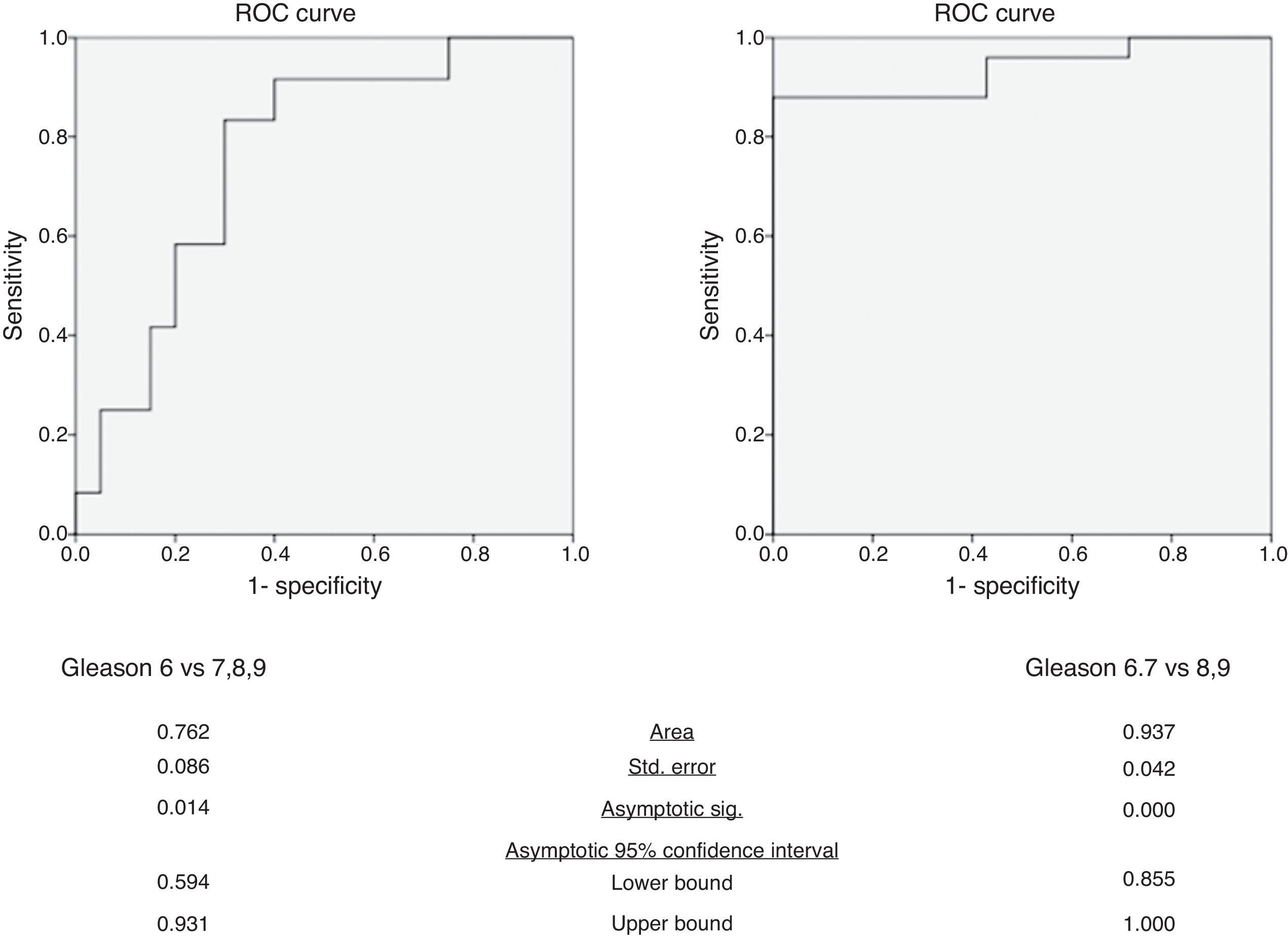
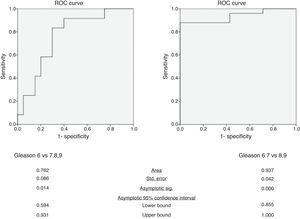
In the differentiation of tumors with a Gleason score of 6 from those with a Gleason score of at least 7, mean ADC yielded an AUC of 0.76 (95% confidence interval: 0.59, 0.93) (Fig. 3). The p value of Hosmer–Lemeshow test was 0.789. For an ADC of 0.906×10−3mm2/s, sensitivity and specificity were 83.3% (95% confidence interval: 51.6%, 97.9%) and 70.0% (95% confidence interval: 45.7%, 88.1%), respectively.
In the differentiation of tumors with Gleason scores of 6 or 7 from those with a Gleason score of at least 8, mean ADC yielded an AUC of 0.94 (95% confidence interval: 0.86, 1.00) (Fig. 3). The p value of Hosmer–Lemeshow test was 0.965. For an ADC of 0.649×10−3mm2/s, sensitivity and specificity were 88.0% (95% confidence interval: 68.8%, 97.5%) and 100% (95% confidence interval: 59.0%, 100%), respectively.
DiscussionThis study shows a significant negative correlation between ADC values and Gleason scores, which is consistent with other studies like those of Bittencourt et al.6 or Verma et al.12 This manuscript also shows that mp-MRI on a 1.5-T magnet without ERC allows a good discrimination between Gleason scores of 6 and more than 6, and an excellent discrimination between Gleason scores of 7 or lower and more than 7. We were able to provide cut offs with good sensitivity and specificity levels between different Gleason scores, which may constitute an indirect way of determining biologic aggressiveness of the tumor, with consequent treatment implications. However, it remains unclear how to use ADC quantification across imaging platforms and institutions since some deviations have been shown, with greater variation reported at 1.5-T. These variations are probably multifactorial and depend on gradient systems, coil systems, pulse sequence design, imaging parameters, susceptibility artifacts, and postprocessing. Furthermore, when comparing 3.0-T and 1.5-T magnets, slightly higher values have been obtained at the former.13–15 By now, the authors believe that it is not possible to get a reliable cut-off ADC value for malignancy that may be used across different systems. ADC values should then be obtained and compared across different examinations using the same protocol and magnet, which may be particularly useful in set of active surveillance.
Patients with Gleason scores of 6 are stratified as low risk patients. Depending on clinical and laboratorial findings, a protocol of active surveillance may be performed. Serum PSA (which should be less than 10) is the most important laboratorial feature, but PSA velocity and PSA density may also be considered. Since the randomized biopsy tends to underestimate the Gleason score, mp-MRI seems to have an important role in non-invasive aggressiveness assessment and, when combined with serum PSA, may be able to accurately estimate the risk. Many centers consider other features like the percentage or number of positive scores to estimate tumor volume and select patients for active surveillance.16,17 New studies should be developed in order to evaluate if mp-MRI findings better estimates tumor volume than these biopsy features.
According to our results, mp-MRI is able to exclude high-risk patients, which are those with Gleason scores of at least 8. However, the distinction between low and intermediate risk was not so good (sensitivity of 83%). This difference was already expected since an overlap between ADCs on Gleason scores of 6 and 7 had been apparent. As a result, it seems not to be possible to obviate a guided biopsy whenever ADC values decrease. A decrease on ADC is probably related to an increase in the histological aggressiveness and should be considered as tumor progression. Other features should obviously be evaluated, like the size, the existence of new foci, changes on post-gadolinium dynamic patterns, and signs of extra-prostatic extension.
It is not possible for all institutions to acquire the most recent technology and accompany the evolution on protocols, so each department should adapt technical parameters according to the magnet and software characteristics, and evaluate its own diagnostic accuracy. In the particular set of ADC measurements, the results should be compared with those derived from similar MR systems and protocols.13,14 According to some authors, optimal mp-MRI on a 1.5T magnet requires the use of an endorectal coil (ERC) combined with a pelvic phased-array coil (PPA) in order to produce high signal to noise ratios (SNR) and therefore improve the image resolution and acquisition speed. However, there is no consensus about this item and others suggest that the use of ERC might not be mandatory for tumor detection and localization. It may consequently save time and costs, and cause the patient less discomfort.2,18–21 Our results are concordant with the most recent studies with and without ERC. Therefore, we conclude that mp-MRI on a 1.5-T magnet without ERC is highly specific and sensible, and may be used for assessment of tumor aggressiveness.
Our study had some limitations. First, it was retrospective. Second, our results may not apply to a large patient population, because we have only considered patients who underwent radical prostatectomy and only 7 patients (21.4%) had a Gleason score of 8 or higher. Third, these results may only apply to peripheral tumors. Considering central and transitional tumors show distinct behavior on DWI, a specific study should be performed. Fourth, our ADCs were derived from single slice-based ROIs. Moreover, only a ROI was placed within each tumor foci (in the darkest area). So, we performed a visual, qualitative evaluation, and did not quantitatively study the entire foci. Fifth, the slice thicknesses of prostatectomy step-sections and ADC maps were not exactly the same.
Overall, our study shows results similar to other manuscripts. Nevertheless, it is important to emphasize that limitations in acquiring the most recent technology should not constraint protocol evolution and adaptation by each institution. Sustained analysis with internal technology should be performed in order to assess diagnostic accuracy and validate technical protocols. In summary, our results show that mean ADC values are inversely correlated with prostatectomy Gleason scores of peripheral tumors. In combination with PSA levels, mean ADC values may allow a correct assessment of the patient risk for treatment and active surveillance purposes, on a 1.5T magnet without ERC.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Ana Luísa Papoila, PhD, and Marta Alves, MD, for guidance and help with statistical analysis.