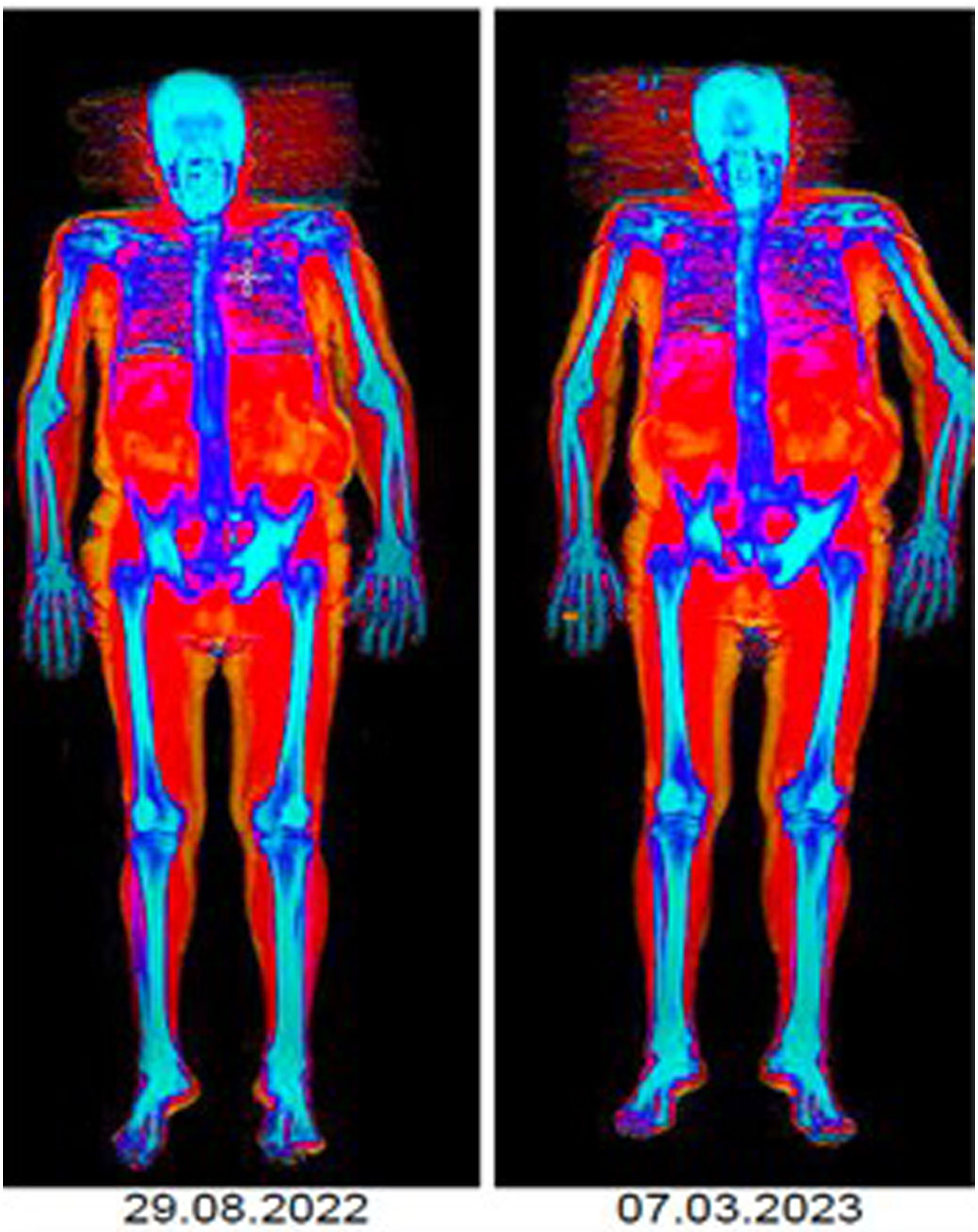
Prostatic carcinoma (PC) is a frequent neoplasm in elderly patients. Although androgen deprivation is associated with survival benefits, it is also related to adverse effects such as osteoporosis, frailty, or sarcopenia, which can negatively affect the patient’s quality of life. This study aims to quantify and evaluate the prevalence of osteoporosis, frailty, or sarcopenia in elderly PC patients before and after androgen deprivation. We present data from an interim analysis.
Materials and methodsPROSARC is a national (Spain) prospective observational study (May-2022–May-2025) still in progress in 2 hospitals. It includes patients with high-risk PC, aged ≥70 years, non-candidates for local treatment and scheduled to start androgen deprivation therapy. The following variables are analyzed: comorbidity, frailty (Fried frailty phenotype criteria), osteoporosis, sarcopenia (EWGSOP2), fat mass and muscle mass, before treatment and after 6 months of follow-up.
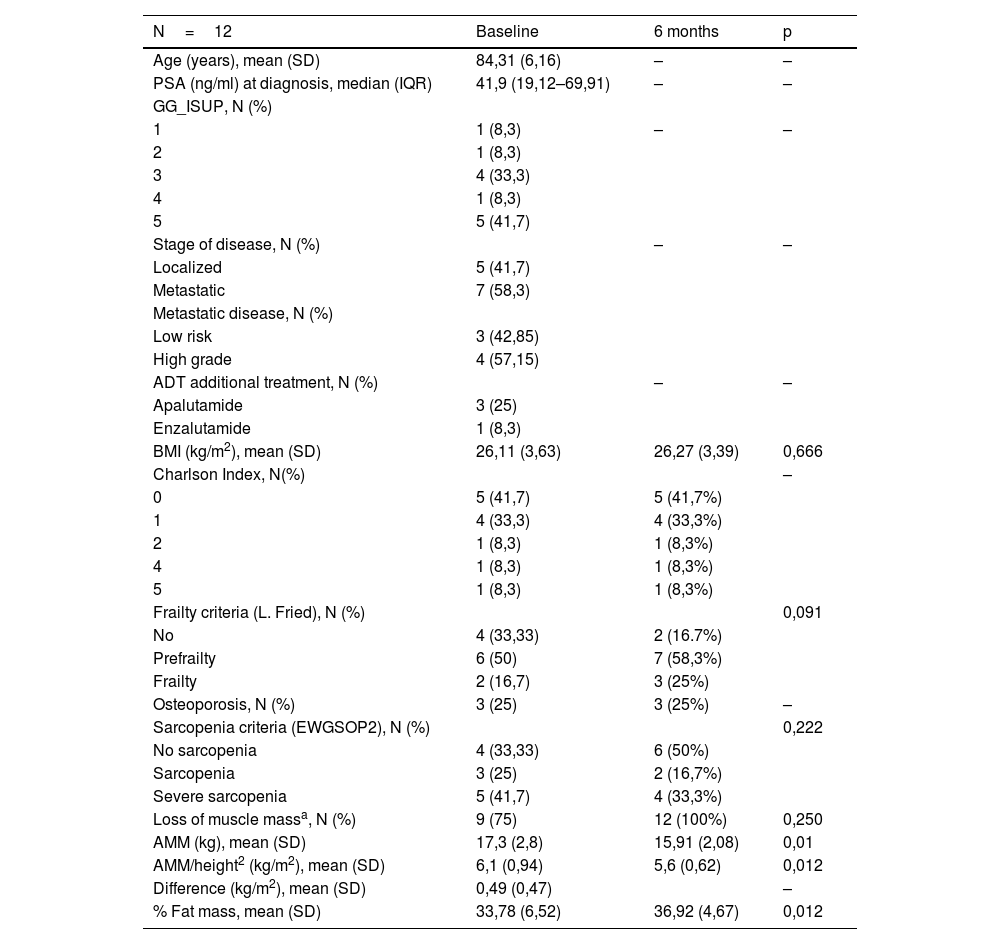
ResultsA 6-month follow-up was completed by 12/25 included patients (mean age, 84 years), with a high baseline prevalence of pre-frailty/frailty (67.7%), sarcopenia (66.7%) and osteoporosis (25%). Treatment did not significantly alter these variables or comorbidity. We observed changes in body mass index (p=0.666), decreased mean value of appendicular muscle mass (p=0.01) and increased percentage of fat mass (p=0.012).
ConclusionIn patients with high-risk PC, advanced age and a considerable prevalence of osteoporosis, frailty and sarcopenia, androgen deprivation (ADT; 6 months) produces decreased muscle mass without impact on the incidence of the known adverse effects of androgen deprivation.
El carcinoma prostático (CP) es una neoplasia frecuente en pacientes de edad avanzada. La privación androgénica, con beneficio en supervivencia, se relaciona con efectos adversos tales como osteoporosis, fragilidad o sarcopenia, que pueden impactar negativamente en la calidad de vida del paciente. Este estudio pretende cuantificar y evaluar el estado de osteoporosis, fragilidad o sarcopenia en pacientes con CP de edad avanzada antes y después de la privación androgénica. Presentamos datos de un análisis intermedio.
Materiales y métodosEstudio nacional (España) observacional prospectivo, PROSARC, aún en marcha (mayo del 2022-mayo del 2025) en 2 hospitales. Incluye a pacientes con CP de alto riesgo, ≥ 70 años, no candidatos a tratamiento local e inicio programado de privación androgénica. Se analizan las siguientes variables: comorbilidad, fragilidad (criterios de Fried), osteoporosis, sarcopenia (EWGSOP2), masa grasa y masa muscular, antes del tratamiento y tras 6 meses de seguimiento.
ResultadosCompletaron un seguimiento de 6 meses 12/25 pacientes incluidos (edad media, 84 años), con una elevada prevalencia basal de prefragilidad/fragilidad (67,7%), sarcopenia (66,7%) y osteoporosis (25%). El tratamiento no varió significativamente estas variables ni la comorbilidad. Se observaron cambios en el índice de masa corporal (p=0,666), reducción del valor medio de la masa muscular apendicular (p=0,01) e incremento del porcentaje de masa grasa (p=0,012).
ConclusiónLa privación androgénica (6 meses) en pacientes con CP de alto riesgo, edad avanzada y una prevalencia considerable de osteoporosis, fragilidad y sarcopenia, produce disminución de masa muscular sin que ello repercuta en la incidencia de los efectos adversos de la privación androgénica estudiados.
Artículo
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora