Allergen-specific immunotherapy balances the Th2-biased immunity towards Th1 and Treg responses. Adjuvants are used in allergen preparations to intensify the immune responses. The increased prevalence of allergies in developed societies has been associated with decreased microbial load during childhood. This has initiated a search for microbial structures to be used as adjuvants. Our study has shown that a synthetic triacedimannose (TADM) may suppress the Th2-type allergic inflammatory response. The aim of this study was to compare the properties of TADM with capacities of other adjuvants, CpG ODN and MPL, to modulate cytokine production in PBMC and regulate sensitisation in an OVA-sensitised mouse asthma model.
MethodsThe effects of TADM were studied in vitro on birch stimulated PBMC cultures of birch allergic rhinitis patients with other known adjuvants. Cytokines in supernatants were measured by Luminex. Effects of TADM were analysed in vivo in a mouse model of OVA-induced allergic asthma by analysing BAL, cytokine mRNA and serum antibodies.
ResultsTADM was the only adjuvant that significantly suppressed the production of all birch induced Th2-type cytokines. In a murine model, TADM significantly suppressed the specific IgE production and enhanced IFN-γ production.
ConclusionsTADM suppresses the birch allergen induced Th2-type cytokine responses in allergic subjects more efficiently than the two other adjuvants, MPL and CpG ODN. TADM is immunomodulatory also in vivo and decreases the IgE levels and increases the IFN-γ responses in a murine model. These results suggest that TADM may be a promising candidate for novel adjuvants in immunotherapy.
In allergic subjects, an allergen encounter leads to a T helper 2 (Th2)-type immune response followed by a subsequent immunoglobulin E (IgE)-mediated hypersensitivity reaction and further allergic inflammation1. An effective treatment for allergic diseases is allergen-specific immunotherapy (SIT) which balances the Th2-biased immunity towards Th1 and T regulatory (Treg) responses.1 Since the prevalence of allergic diseases is increasing, the requirements for SIT are also high, some of them being optimal potency with minimal disadvantages. To intensify the immune response and to shorten the injection regimens research has focused on adjuvants conjugated to allergen preparations.2 Adjuvants target the allergens to tolerogenic antigen presenting cells (APCs) and enhance the recognition of allergens. Tolerogenic APCs promote and maintain immunologic tolerance by inducing cell anergy and the differentiation of Treg cells. Adjuvants modify also Th differentiation leading to pro- or anti-inflammatory immune responses according to the properties of adjuvants. The following immune responses during SIT can be closely modulated by selecting the adjuvant carefully. The optimal adjuvant would favour Th1 and Treg pathways while simultaneously suppressing Th2 responses.3
The increased prevalence of atopic allergies in developed societies has partly been associated with high hygiene and decreased microbial load during childhood, diminishing the natural Th1 responses leading to enhanced Th2-type immunity.4 This has initiated a search for microbial structures to be used as adjuvants. These adjuvants would induce a local Th1-type environment and reduce allergen-induced Th2 responses, thereby suppressing allergic inflammation.3 Several structures from microbes, including immunostimulatory oligonucleotides (CpG ODN) and monophosphoryl lipid A (MPL), induce Th1 immune responses and downregulate allergen-induced Th2 responses.5,6 However, none of these molecules have established a firm position as allergy adjuvants in clinical use.
Another adjuvant used in immunotherapy since 1938 is aluminium hydroxide (alum).7 It has been shown to downregulate Th2 responses and enhance the contact between an APC and an allergen.8 Paradoxically, it promotes Th2-type reactions which lead to undesired induction of IgE production.8 In fact, this is why alum is widely used as a stimulator of allergic inflammation in murine models. Therefore, other alternatives to immunologic adjuvants in allergy preparations have been explored and proposed.
Microbes, including fungi, are nowadays widely demonstrated as Th1-inducing structures. Fungal cell wall components, and especially mannan of Candida albicans (C. albicans), induce Th1 responses.9–11 Therefore we have previously chemically synthesised and screened a range of oligosaccharide molecules mimicking the C. albicans cell wall mannans rich in β-(1,2)-mannose structures.12–14 According to the literature and our former studies, one linkage type, called β-(1,2)-linkage, relates to immunostimulatory properties of a mannoside.12–14 We recently discovered in a pilot study that a trivalent acetylated mannobiose with β-(1,2)-linkages, 1,2,3-tris(1-((2,3,4,6-tetra-O-acetyl-β-D-mannopyranosyl)-(1→2)-(3,4,6-tri-O-acetyl-α-D-mannopyranosyloxyethyl))-4-(methyl-1-oxy)triazolyl)propane, induced Th1-type immune responses in healthy subjects and suppressed birch induced interleukin-4 (IL-4) and IL-5 responses in birch allergic subjects.14 This trivalent acetylated mannobiose will hereafter be referred to as triacedimannose (TADM). The objective of this study was to compare the immunomodulatory properties of potent adjuvants, TADM, CpG ODN and MPL, in human peripheral blood mononuclear cells (PBMC) of allergic subjects and to characterise the modulatory effects of TADM on allergen-induced inflammatory responses in a murine asthma model. The goal of this study was to evaluate whether TADM could serve as an adjuvant for future SIT.
Materials and methodsStudy subjectsDuring the pollen season, 26 adult birch allergic subjects with allergic rhinoconjunctivitis (22 females and 4 males) were enrolled in the study (mean age 37.7 years, SD 11.1 years; mean birch-specific IgE (Immunocap, Thermo Fisher Scientific Phadia, Uppsala, Sweden) 25.2kU/l, SD 27.0kU/l). All samples were taken after informed consent. The study was approved by the local ethics committee.
AdjuvantsTADM was synthesised as previously described.14 Synthetic MPL and an active CpG ODN (tlrl-2006) and a control CpG ODN (tlrl-2006c) were purchased from Invivogen (San Diego, CA, USA). The active CpG ODN had a sequence of 5′-TCG TCG TTT TGT CGT TTT GTC GTT-3′, identical to one used in a previous study.15
PBMCThe PBMC were isolated by Ficoll-Paque density gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare Bio-Sciences AB, Uppsala, Sweden) from heparinised blood samples from study subjects during pollen season. The PBMC were washed twice with Hanks’ balanced salt solution (HBSS) buffered with NaHCO3 (pH 7.4) and resuspended in RPMI-1640 culture medium (Invitrogen Co., Carlsbad, CA, USA) supplemented with 5% autologous serum, 2.5mM l-glutamine (Sigma–Aldrich Co., St. Louis, MO) and 100μg/ml gentamycin sulphate (Biological Industries Ltd., Kibbutz Beit Haemek, Israel) and applied on 48-well flat-bottomed cell culture plates (Costar, Corning Inc., New York, USA) at a density of 106/ml. Cells were stimulated with various concentrations of adjuvants. Medium alone served as an unstimulated control. All incubations were performed at +37°C in humidified atmosphere with 5% CO2.
Stimulations of PBMC with adjuvantsEffects of adjuvants on birch-pollen induced cytokine productions were studied with PBMC from birch-allergic subjects during (n=26) the pollen season. Cells were stimulated with two concentrations of TADM (20, 200μg/ml), MPL (10μg/ml), active and control CpG ODN (5μg/ml) co-cultured in the presence of birch allergen (50μg/ml, Betula verrucosa, Bet v, Aquagen, ALK-Abelló A/S, Hørsholm, Denmark). The used concentrations of TADM were chosen according to a previous study.14 The used concentrations of MPL and CpG ODN were based on manufacturer's instructions (Invivogen). Supernatants from cultures performed in duplicate were collected 72h after beginning of the stimulation and stored at −70°C.
Cytokine production of PBMCThe cytokines in supernatants (interferon-γ (IFN-γ), interleukin-4 (IL-4), IL-5, IL-10, IL-13 and tumour necrosis factor (TNF)) were measured with high-sensitivity human cytokine Lincoplex kits (LINCO Research, St. Charles, MO, USA). The assays were performed in accordance with the manufacturer's protocol by employing Luminex technology.
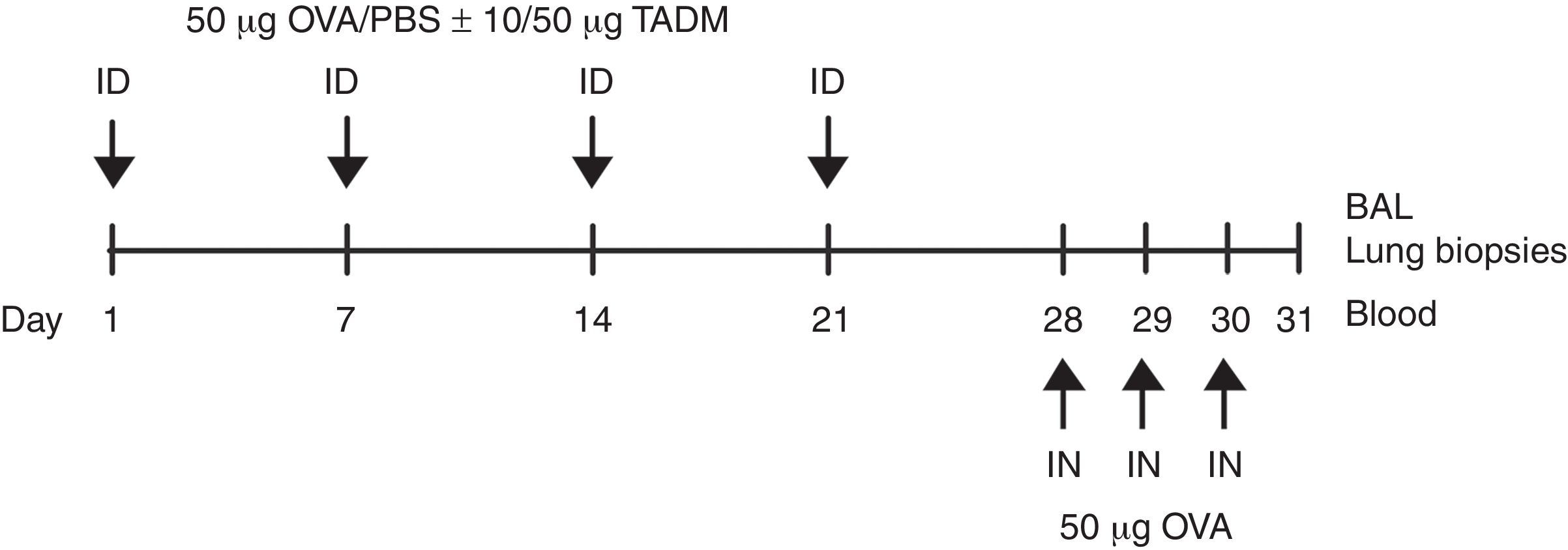
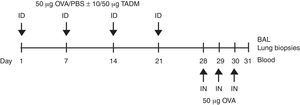
Mice and their treatment6–8 week-old female BALB/c mice (NOVA-SCB AB, Sollentuna, Sweden) (n=eight per group) were maintained on ovalbumin (OVA)-free diets and water ad libitum. The mice were anaesthetised (isoflurane, Abbott Laboratories Ltd., Queensborough, UK) and their backs were shaved and tape-stripped (Tegaderm 3M Health Care, St. Paul, MN, USA) three times before the injections to induce a skin injury. Weekly intradermal sensitisations were made with 50μg of OVA (Sigma–Aldrich) in 100μl, or with PBS (control group) for four weeks. TADM (10 or 50μg) was intradermally injected together with OVA or PBS. On days 28, 29 and 30 all mice were anaesthetised and intranasally challenged with OVA (50μg in 50μl of PBS). After 24h, the mice were killed with isoflurane, and samples (blood, bronchoalveolar lavage (BAL) fluids and lung biopsies) were taken for subsequent analysis (Fig. 1).
BAL cells and lung histology of miceBAL inflammatory cells as well as H&E and PAS stained lung biopsies were obtained and prepared as earlier described.16 Finally, cells were counted under light microscopy (Leica DM 4000B, Wetzlar, Germany).
RT-PCR analysis of lung tissueLung biopsies were quick-frozen and kept at −70°C for later RNA isolation. Samples were homogenised with Lysing Matrix D tubes (MP Biomedicals, Solon, OH, USA) and TRIsure reagent (Bioline, London, UK) in a FastPrep FP120-machine (BIO 101, Thermo Fisher Scientific, Waltham, Mass, USA). Total RNA extraction was performed according to the manufacturer's instructions (TRIsure). Complementary DNA synthesis and real-time PCR with 7500 Fast Real-time PCR System and SDS Software v.1.40 (Applied Biosystems, Foster City, CA, USA) were made as previously described.17 Normalisation control in the qPCR is 18S as earlier described.15 The gene expression was normalised with endogenous 18S rRNA and calculated by the comparative CT-method according to the instructions. Results are shown as relative units (RU).
Serum antibodies in mice seraOVA-specific IgE (capture ELISA) and IgG2a (straight ELISA) were analysed with ELISA as earlier described with small modifications.18 Coating concentrations were 2μg/ml rat anti-mouse IgE for OVA-specific IgE and 2μg/ml OVA for OVA-specific IgG2a. Serial dilutions of sera were 1/20, 1/60 and 1/120 for OVA-specific IgE and 1/200, 1/600, 1/1800 and 1/5400 for OVA-specific IgG2a. Bound OVA-specific IgE was detected with biotin-conjugated OVA (diluted 1/1000), which was prepared with NHS-LC-Biotin kit (Pierce, Thermo Scientific, Rockford, IL, USA). Monoclonal antibodies and streptavidin-horseradish peroxidase were obtained from BD (San Diego, CA, USA) and substrate solution from KPL, Inc. (Gaithesburg, MD, USA).
StatisticsWilcoxon's Signed Rank test was used to test statistical significance in PBMC experiments. The statistical significance was calculated comparing the production of cytokines after stimulation with any given adjuvant to the level before stimulation. Single mice groups were compared by the nonparametric Mann–Whitney U-test using GraphPad Prism software (v.5, GraphPad Software Inc., La Jolla, CA, USA). Data are expressed as mean±SEM and P-values of <0.05 are considered statistically significant.
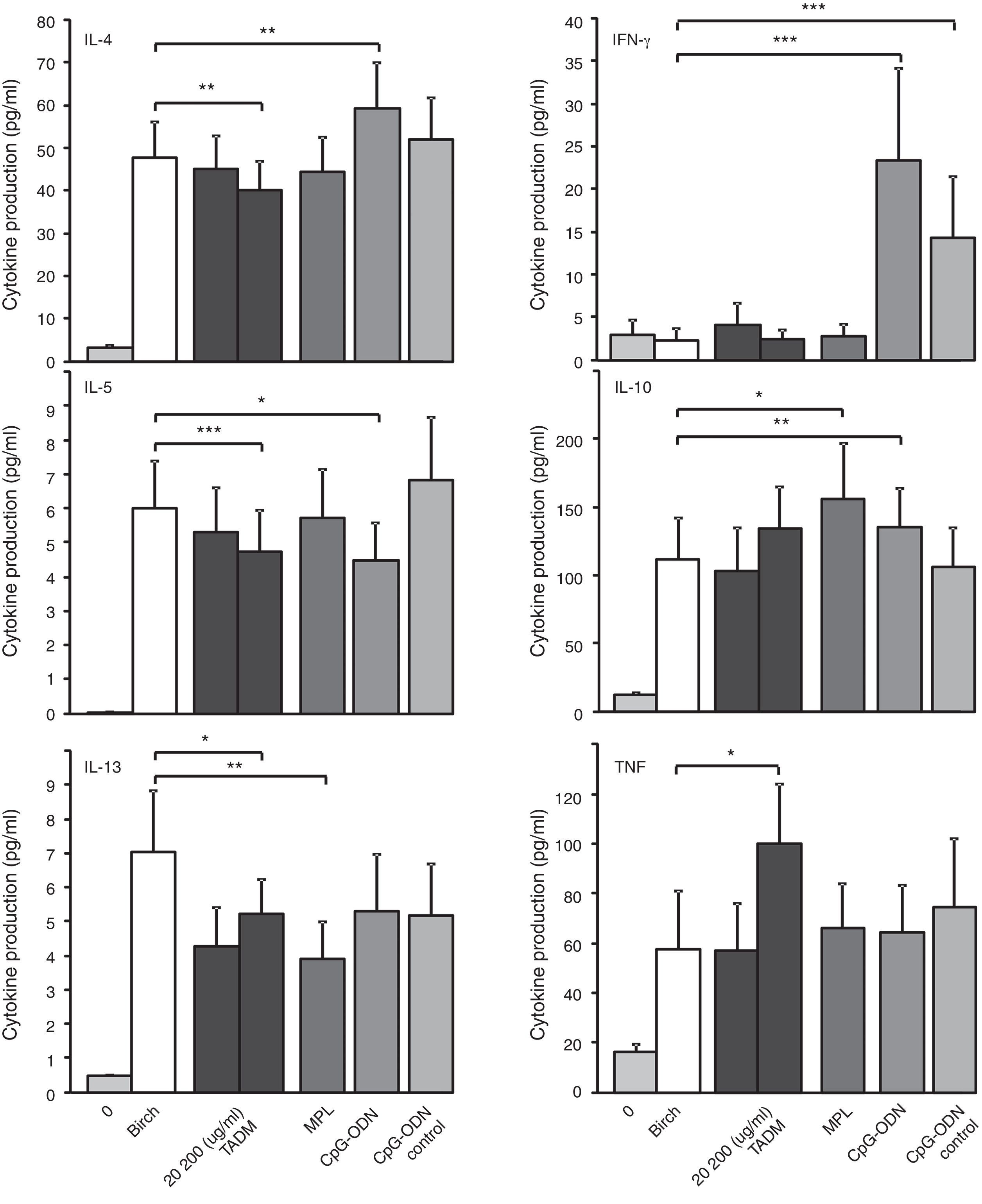
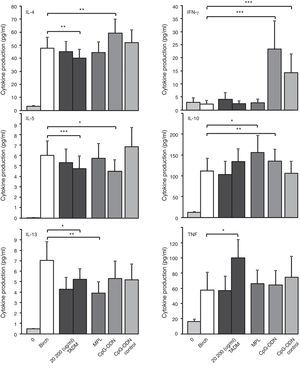
ResultsEffects of TADM, MPL and CpG ODN on birch-induced cytokine responses of PBMC from allergic subjects during pollen seasonThe effects of TADM, MPL and CpG ODN on allergen (Bet v) induced cytokine responses in PBMC cultures of 26 birch allergic rhinitis patients are presented in Fig. 2. Stimulation with birch-induced statistically significant responses of Th2 cytokines IL-4, IL-5 and IL-13. Only TADM suppressed birch-induced production of all three studied Th2 cytokines (IL-4, IL-5 and IL-13), whereas CpG ODN suppressed only IL-5 and MPL only IL-13 production. CpG ODN increased the birch-induced IL-4 and IFN-γ productions. TNF production was enhanced by TADM and IL-10 production by MPL and CpG ODN.
Effects of TADM (20, 200μg/ml), MPL (10μg/ml), CpG ODN and control CpG ODN (5μg/ml) on birch (50μg/ml) induced cytokine responses (pg/ml; IFN-γ, IL-4, IL-5, IL-10, IL-13, TNF) in PBMC of birch allergic subjects (n=26) during pollen season after 72h stimulation measured by Luminex technology. Data represent mean±SEM, *P<0.05, **P<0.01, ***P<0.001.
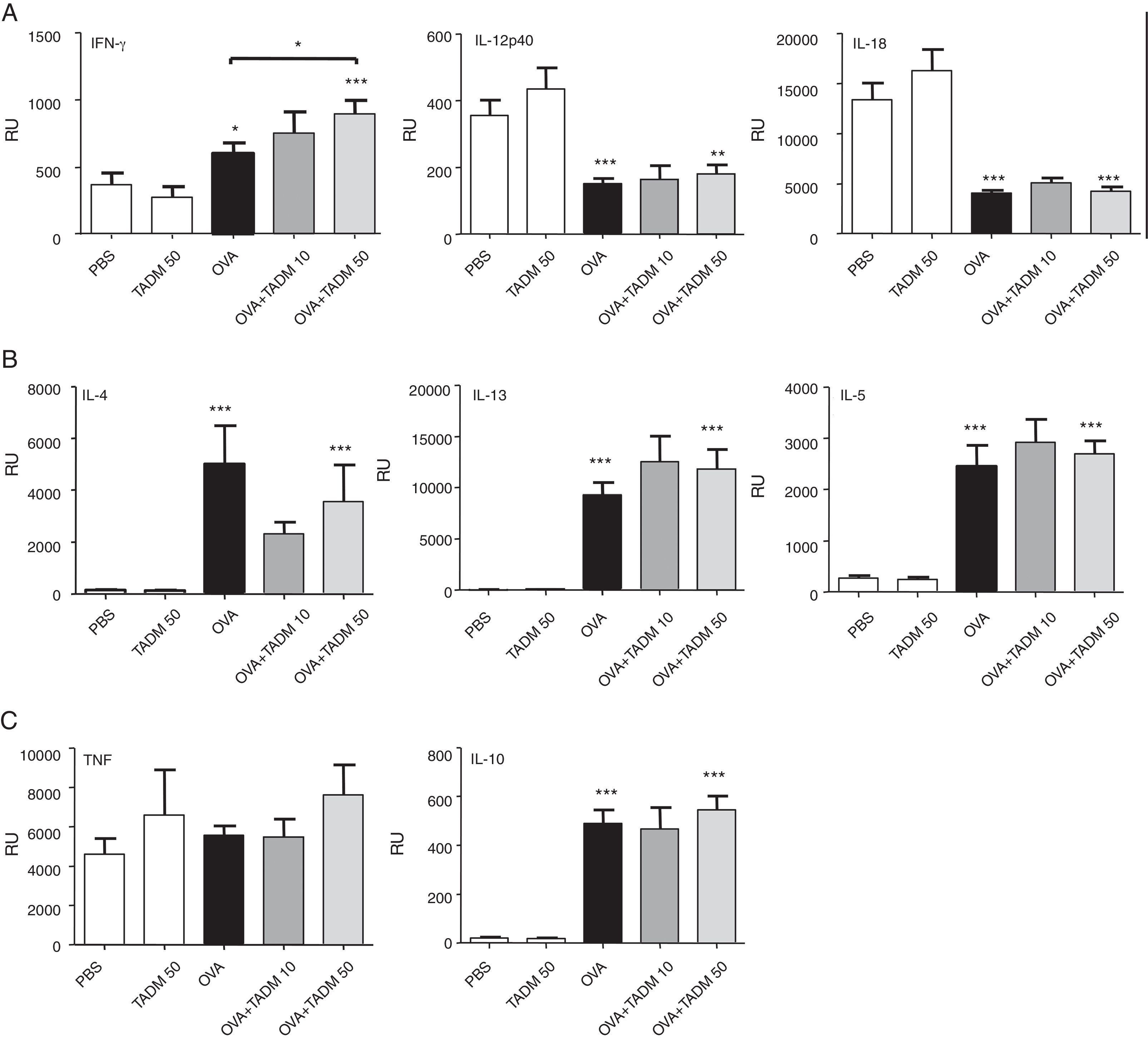
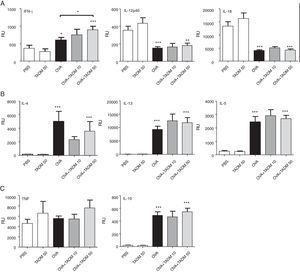
Cytokine profiles of lung tissues reflect the main immune responses of mice. OVA treatment induced a mild mRNA expression of IFN-γ, while expression of other Th1-type cytokines decreased (Fig. 3A), and all studied Th2 cytokines significantly increased (Fig. 3B) in mice lungs. TNF mRNA levels remained at the baseline level, whereas IL-10 mRNA expression significantly increased in mice lungs after OVA exposure (Fig. 3C). While the lung expression of different cytokine mRNAs was similar in PBS and TADM mice, TADM significantly increased IFN-γ mRNA expression (Fig. 3A). In addition, TADM non-significantly decreased the mRNA expression of IL-4 (Fig. 3B).
Cytokine mRNA expression in lung biopsies of BALB/c mice. Th1-type (A), Th2-type (B), pro-inflammatory (C) and regulatory (C) cytokines (relative units; RU). Data represent n=8 mice per group, mean±SEM; *P<0.05, **P<0.01, ***P<0.001. OVA, ovalbumin; TADM, triacedimannose (one dose 10μg or 50μg/mice).
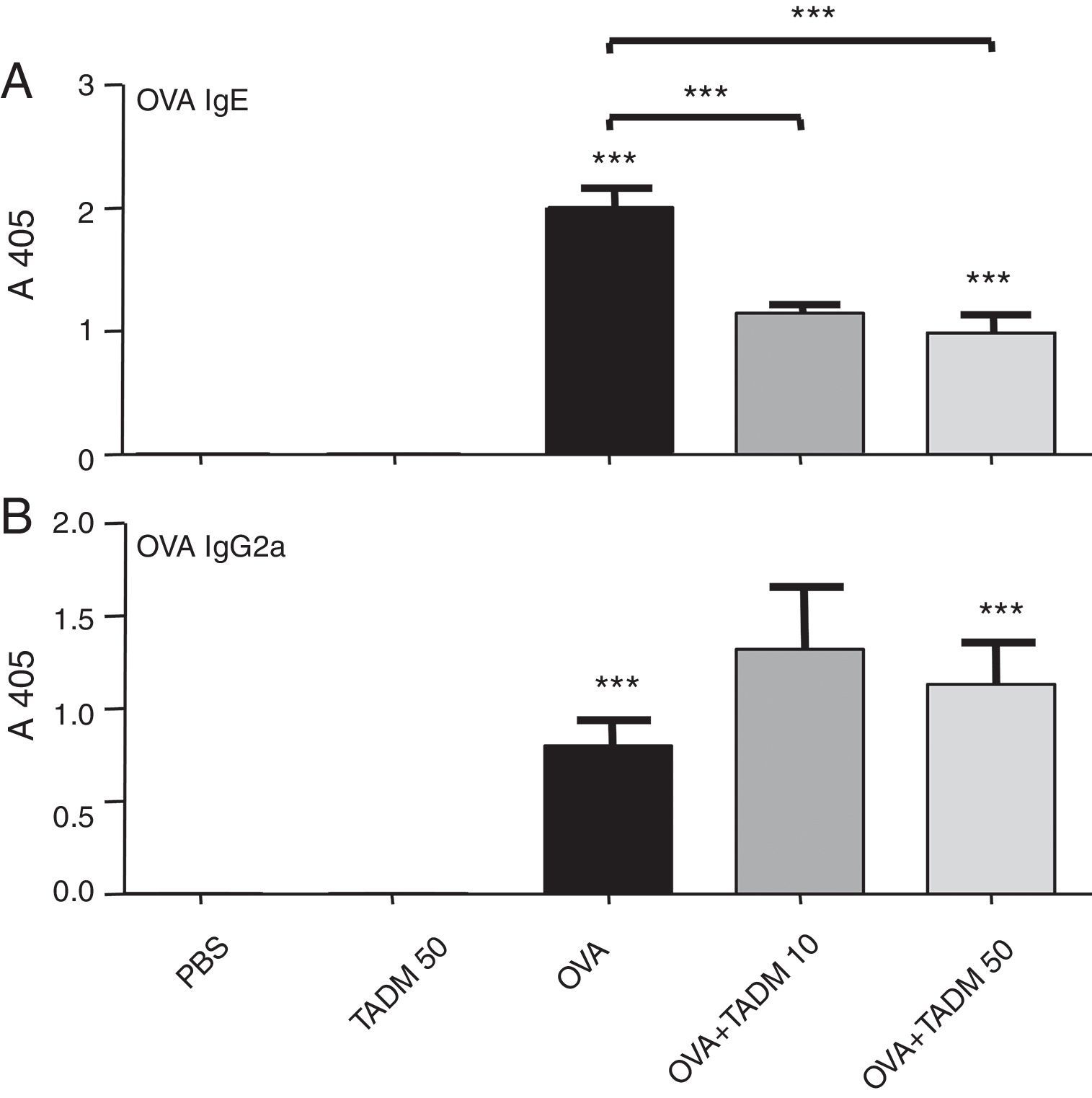
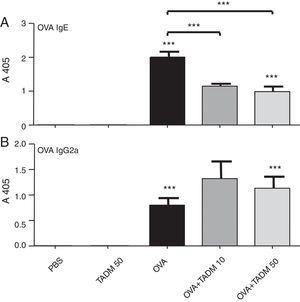
OVA specific IgE and IgG2a antibodies were found after OVA treatments in mice sera (Fig. 4). TADM significantly decreased the OVA specific IgE levels in OVA mice (Fig. 4A), whereas the OVA specific IgG2a levels increased (Fig. 4B). There were no differences between the control groups (PBS vs. TADM).
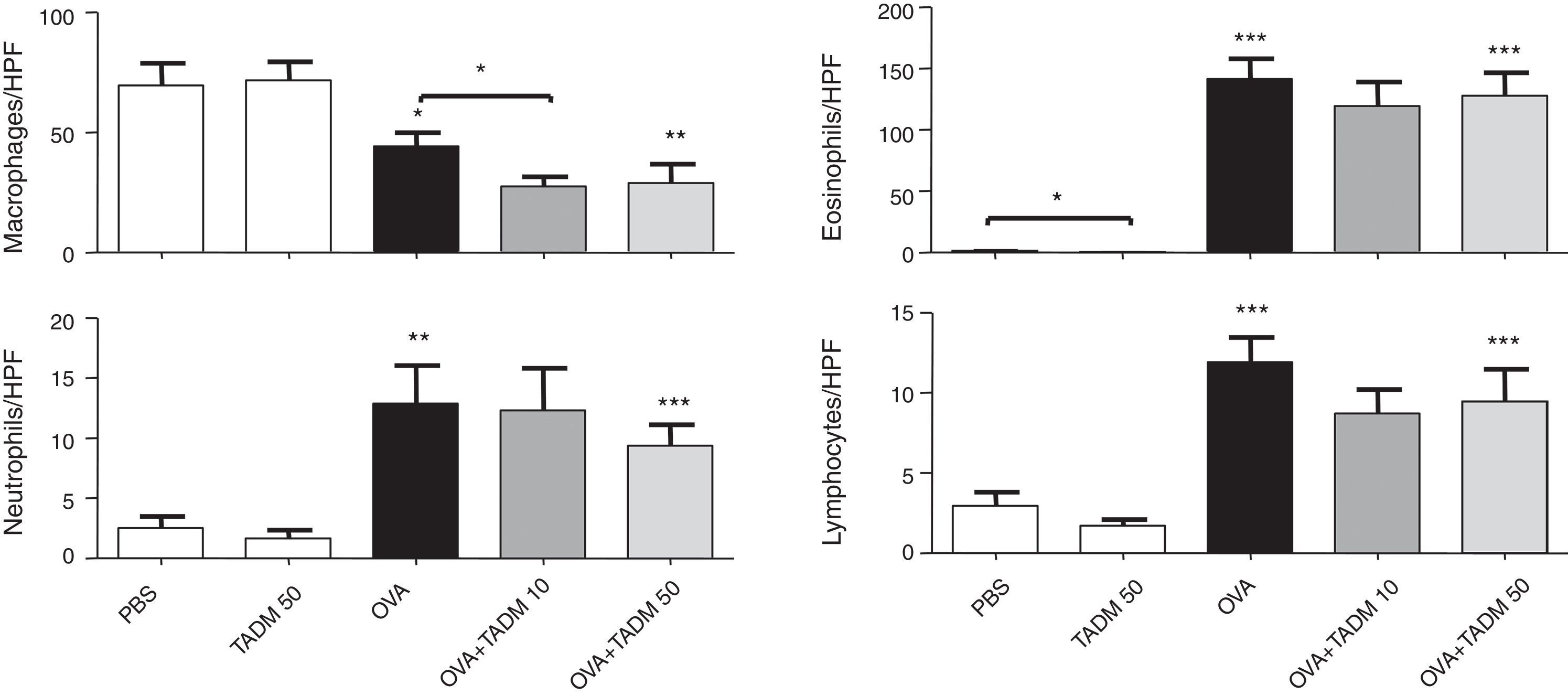
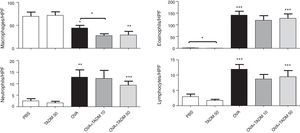
Inflammation in mice airwaysOVA exposure and challenge induced inflammation in the murine airways. The number of inflammatory cells increased significantly in H&E stained lung samples as well as the amount of cells producing mucus (PAS cells) according to PAS staining. TADM had no effect on the infiltration of inflammatory cells to lungs, although the number of PAS cells decreased slightly (about 10%) with lower TADM concentration in OVA treated mice (results not shown). OVA treatment also significantly changed the cell distributions in BAL fluids, decreasing the macrophage cell counts and increasing the amounts of inflammatory cells, especially eosinophils (Fig. 5). In general, TADM treatment could not influence cell types found in BAL fluids, although the number of macrophages declined in the OVA sensitised and challenged mice (Fig. 5). In addition, eosinophil counts were lower in the TADM control group compared to PBS controls (Fig. 5).
Inflammatory cells (macrophages, neutrophils, eosinophils, lymphocytes) in bronchoalveolar lavage (BAL) fluids. Cells were counted per high-power field (HPF). Data represent n=8 mice per group, mean±SEM; *P<0.05, **P<0.01, ***P<0.001. OVA, ovalbumin; TADM, triacedimannose (one dose 10μg or 50μg per mice).
In this study novel possibilities to modulate allergic immune responses were investigated in vitro and in vivo by employing a synthetic carbohydrate construct, TADM, as an immunomodulator. We have previously screened several synthetic analogues of C. albicans cell wall mannan oligosaccharides and found that TADM induces IL-10 and IFN-γ in PBMC of healthy subjects and suppresses allergen induced IL-4 in PBMC of allergic subjects.12–14 In this study TADM was compared and benchmarked against the previously established adjuvants, CpG ODN and MPL. Additionally the effects of TADM on the sensitisation and allergic inflammation to OVA were studied in a mouse model of asthma.
To study the capacities of TADM to modulate on-going allergic inflammation we used PBMC retrieved from pollen allergic subjects during pollen season and stimulated these cells with birch allergen. During the pollen season, continuous allergen challenge enhances Th2-type responses and leads to sustained Th2-type immunity.19 This was also clear in our study, showing elevated productions of Th2-type cytokines IL-4, IL-5 and IL-13 in PBMC of allergic subjects during the pollen season. Thereafter, the cell cultures were stimulated with different concentrations of TADM, MPL or CpG ODN, and the produced cytokines were measured. With the concentrations applied here, TADM was the only adjuvant that suppressed all birch-induced Th2-type cytokine productions during the pollen season. The applied concentrations of the earlier established adjuvants (CpG ODN 5μg/mL and MPL 10μg/mL) were selected based on the data provided by the manufacturers and a previous study.15 MPL suppressed IL-13 production and CpG ODN only IL-5 production. During SIT, repeated administrations of the allergen extracts result in suppression of the Th2-type responses (IL-4, IL-5 and IL-13), together with enhancement of the Treg- (IL-10) and Th1-type responses (IFN-γ).20–22 Consistently, similar suppression of the Th2-type immune responses was also observed with TADM.
All three studied adjuvants also induced pro-inflammatory cytokine, TNF, production. It is produced by macrophages and the Th1 cells.23,24 Macrophages have a pivotal role in allergic inflammation since they can either enhance or suppress immune responses depending on the cytokine environment.25 According to the literature, MPL stimulates TNF, IL-10 and IFN-γ production by ligating to Toll-like receptor 4 (TLR-4), which leads to NF-κB transcriptional activity.26 Also the β-(1,2)-linked oligomannosides significantly improve TNF secretion and these structures are recognised by galectin-3.27,28 The simultaneous induction of TNF and the suppression of the Th2-type cytokines observed in this study with TADM might be due to similar activation cascades shifting the immune responses towards Th1- and Treg-cytokines.
In order to analyse the immunomodulatory effects of TADM also in vivo, we intradermally sensitised and intranasally exposed mice to OVA to create an experimental allergic asthma model.29 With this kind of murine model the capacities of TADM to modulate sensitisation as well as the allergic inflammation can be explored. In this study the allergen was OVA, but the principle mechanisms behind the allergic inflammation are the same regardless of the allergen. However, any given murine model mimics humans only poorly, because there are differences between the species varying from the repertoire of TLRs, their specificity to different ligands to the anatomical sizes. The mice were first sensitised by repeated exposures to the OVA allergen. This leads to OVA-specific Th2-type immune responses. Then the mice were challenged with the OVA-allergen to elicit allergic inflammation. Thereafter blood samples, BAL fluids and lung biopsies were taken for analysis. The cytokine profiles of the lung tissues reflect the main immune responses of the mice. The OVA-treated mice developed strong Th2-type responses (IL-4, IL-5, and IL-13) in the lungs with predominantly eosinophilic cell infiltration. In the mice sera, increased amounts of the OVA-specific Th1-type (IgG2a) and Th2-type (IgE) antibodies were measured after allergen challenge. In accordance with our assumptions, the OVA-specific IgE levels decreased significantly with TADM. In addition, an increase in the OVA-specific IgG2 levels was observed. The key elements of SIT are the decreased allergen-specific IgE levels and the increased levels of IgG.30 Allergen-specific IgGs prevent IgE-mediated histamine release, interactions between the allergens and IgE and allergen presentation.31,32 These functions inhibit early-phase responses to allergen challenge.33 Indeed, in previous studies both CpG ODN stimulation and specific immunotherapy with MPL have led to reduced levels of the allergen-specific IgE.5,34
Although strong alterations were observed in the humoral and cytokine environment after stimulation with TADM in the presence of OVA, no considerable effect on the cells in BAL could be seen, except for a slightly decreased number of macrophages. This is partly explained by the unusually strong OVA-induced eosinophilia, which could not be suppressed efficiently by TADM.
Stimulation with a high concentration of TADM significantly increased IFN-γ secretion and decreased IL-4 mRNA expression in the lung biopsies of BALB/c mice. IFN-γ has previously been demonstrated to inhibit Th2 development and Th2-mediated allergic inflammation.35 In addition, the Th1 cells and the produced IFN-γ inhibit IgE production, but stimulate B cell class switching to IgG isotypes.36 Our results showing decreased IgE levels after allergen exposure and stimulation with TADM are in line with the observed diminished IL-4 production, since IL-4 is needed in IgE production.37 During SIT, tolerance to allergens is increased through the immune deviation from Th2 towards Th1 and Treg cells. Similar immune deviation, at least partly, was also observed with TADM in a murine model of asthma.
In this study we have characterised the immunomodulatory properties of a novel adjuvant candidate, TADM, both in vitro and in vivo. Stimulation with birch pollen induced strong Th2-type immune responses in PBMC of allergic subjects, and these responses could be suppressed efficiently by TADM. In addition, TADM decreased the specific IgE levels and increased the IFN-γ mRNA responses in an OVA-sensitised murine model of asthma. The immunomodulatory properties of TADM presented here are in accordance with our previous study suggesting a possible role for TADM as an adjuvant candidate for immunotherapy.14 Further studies are in progress to further evaluate the properties of TADM as a potent immunotherapeutic adjuvant in SIT.
Authors contributionsThe experiments of this study were conceived and designed by KM, MarL, HA, RL and JS. Experiments were done by KM, CM, MarL, RP, HW and MaiL. Data were analysed and interpreted by KM, MaiL, HA, HW, RL and JS. KM, MaiL, RL and JS wrote the article. All authors were involved in critical revision of this article.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
This study was supported by grants from The Finnish Society of Allergology and Immunology, Allergy Research Foundation of South-Western Finland, Finnish Allergy Research Foundation, The University Central Hospital of Turku and the Academy of Finland (project numbers 122126, 121334, and 121335). Leena Kavén-Honka is acknowledged for her technical assistance.