Currently, mankind is afflicted with diversified health issues, allergies being a common, yet little understood malady. Allergies, the outcome of a baffled immune system encompasses myriad allergens and causes an array of health consequences, ranging from transient to recurrent and mild to fatal. Indoor allergy is a serious hypersensitivity in genetically-predisposed people, triggered by ingestion, inhalation or mere contact of allergens, of which mite and cockroaches are one of the most-represented constituents. Arduous to eliminate, these aeroallergens pose constant health challenges, mostly manifested as respiratory and dermatological inflammations, leading to further aggravations if unrestrained. Recent times have seen an unprecedented endeavour to understand the conformation of these allergens, their immune manipulative ploys and other underlying causes of pathogenesis, most importantly therapies. Yet a large section of vulnerable people is ignorant of these innocuous-looking immune irritants, prevailing around them, and continues to suffer. This review aims to expedite this field by a concise, informative account of seminal findings in the past few years, with particular emphasis on leading frontiers like genome-wide association studies (GWAS), epitope mapping, metabolomics etc. Drawbacks linked to current approaches and solutions to overcome them have been proposed.
Allergies, the pathologic manifestations originated due to a trigger-sensitised immune system have emerged as a bane of current times. Though allergies as health issues are not new to mankind, their severity, owing to pollution, drug abuse, dietary mistakes and chemical exposure is unprecedented in recent times. Considering that allergies erupt when innocuous foreign components are confused as foes by the immune surveillance,1 their dynamics has been extensively studied. The immune system is an intricate network of lymphoid organs, lymphocytes, and cytokines, meant to cordon off against all physical invaders and restore homeostasis. By coordination of its two arms, innate and adaptive immunity, it defends the host against the onslaught of pathogens, intent on wreaking havoc.2 However, due to a multitude of reasons, both genetic and environmental factors, the immune system can undergo deviations and prompt allergic reactions.3 Frequent exposure to the allergens leads to allergic manifestations such as asthma, atopic dermatitis, urticaria, rhinitis, sinusitis and conjunctivitis, to name a few, in susceptible individuals.4 Some allergens can even induce life-threatening conditions such as anaphylaxis. The basal mechanisms of allergic responses vary depending on a host of factors, predominantly the allergens, cross-reactivity with other allergens and host genetic profile. The general pathway has been ramified based on involvement of immunoglobulin E (IgE), that is immediate (IgE-mediated or humoral) or delayed (non-IgE mediated or cell mediated) type. In the former, allergenicity starts on recognition of allergens by antigen presenting cells (APC) like dendritic cells, which polarises Th differentiation towards Th2, causing excess IgE production.5 The allergens bind to IgE, adhered on basophils and mast cells causing their activation, resultant cytokine and chemokine secretion, degranulation and subsequent histamine release.6,7 The cytokines converse with other immune cells and mediate inflammations.8 In delayed type hypersensitivity, T lymphocytes, eosinophils and basophils are recruited to the site of inflammation.9 Most cases of delayed response are associated with drugs.10 Activation of inflammasome in airway, gut and skin epithelium by both modes of sensitisation has been evidenced.
Literature survey reflects that roughly 30% of the world population suffers from allergies.11 Although there exist a plethora of allergens, food, pollen, insects, latex, drugs, cosmetics, metal have been recognised as the most frequent causal agents.12 However, the list of allergens can be overwhelming and our current knowledge on their repertoire is only the tip of the iceberg.13 In current times, allergic instances are rising dramatically, due to many unspecified but suspected reasons. The degrading quality of environment is assumed to be one of the fundamental reasons, as diesel exhaust particle-caused pollen rupture and resultant airway inflammation has been verified.14,15 Also, alterations in lifestyle and dietary habits, higher reliance on processed and preserved foods are assumed to have increased the allergic prevalence.16 A surge in chemical exposure has been incriminated to provoke allergic reactions.17 As allergy is essentially a health exacerbation in atopic individuals, conspired by genetic makeup and environment, it is imperative to decipher both host and allergen aspects and their nexus from the perspective of different allergens. Here, we are discussing the major arthropod-origin indoor allergens, dissecting their diverse standpoints, aimed at their alleviation by promoting awareness.
Arthropods are the largest animal phylum and they sustain a huge diversity.18 It is not in the best interest of human health that several classes of this phylum such as Insecta, Crustacea, Arachnida and Chilopoda have been recognised as allergens.19 In fact, ingestion, inhalation, sting or contact allergy to arthropods poses a significant social, economic, and medical burden across the globe.19 Allergies to house dust mites, cockroaches, hymenopterans, and crustaceans have been frequently documented, as the allergens sensitise and induce IgE-mediated hypersensitivity in predisposed individuals.20,21 Ingestion-caused allergies, as is the case with shrimp, lobsters or crabs can be easily tackled, by simple elimination of the risky diet.22 Sting arthropods can also be avoided by proper precaution.23 However, house dust mites and cockroaches, as indoor allergens, are difficult to get rid of, as these pesky allergens occur in nooks and crannies of houses, and heighten the risk of asthma, dermatitis, sinusitis, rhinitis, and otitis, among other inflammations.24 Not only in houses, but these allergens have been detected in most school samples, which can jeopardise the health of children, rendering them vulnerable to future health consequences.25 The dust mite and cockroach allergies are not restricted to low-income, rural areas in developing countries, but they have been documented in urban areas, in fact more commonly in the latter.26,27 Also, they are not endemic or climate-controlled, but occur globally such as in geographically-segregated countries like Vietnam,26 Thailand,28 Brazil,29 Italy,30 Croatia,27 China,31 to name a few. Eradication of this problem necessitates effective remediation, allergen characterisation and novel therapeutic modalities. To contribute in that direction, by dissemination of knowledge, identification of gaps and stimulation of research, this review has been formulated.
Mite allergens and their mechanismsMites are microscopic arachnids causing allergy worldwide, the major culprits being house dust mite Dermatophagoides pteronyssinus and Dermatophagoides farinae.4,32,33 The two species vary in their allergenic diversity, yet show cross-reactivity.34 Also, Euroglyphus maynei and Blomia tropicalis are common mites in humid parts of the world, which we are not discussing here.35 Also studies on storage mites like Tyrophagus putrescentiae, Lepidoglyphus destructor, Glycyphagus domesticus, Aleuroglyphus ovatus, Acarus siro, Suidasia medanensis have been conducted for richer insights on arthropod-caused allergy, which is beyond the scope of the current discussion.36 Although invisible to unaided eyes, mites are harboured in millions on house furnishings, bedding and clothing, which sustain on discarded human cells.35,37 The recent finding of their occurrence in food articles, like cooking flour, leading to ingestion-related anaphylaxis has raised further concern.38,39
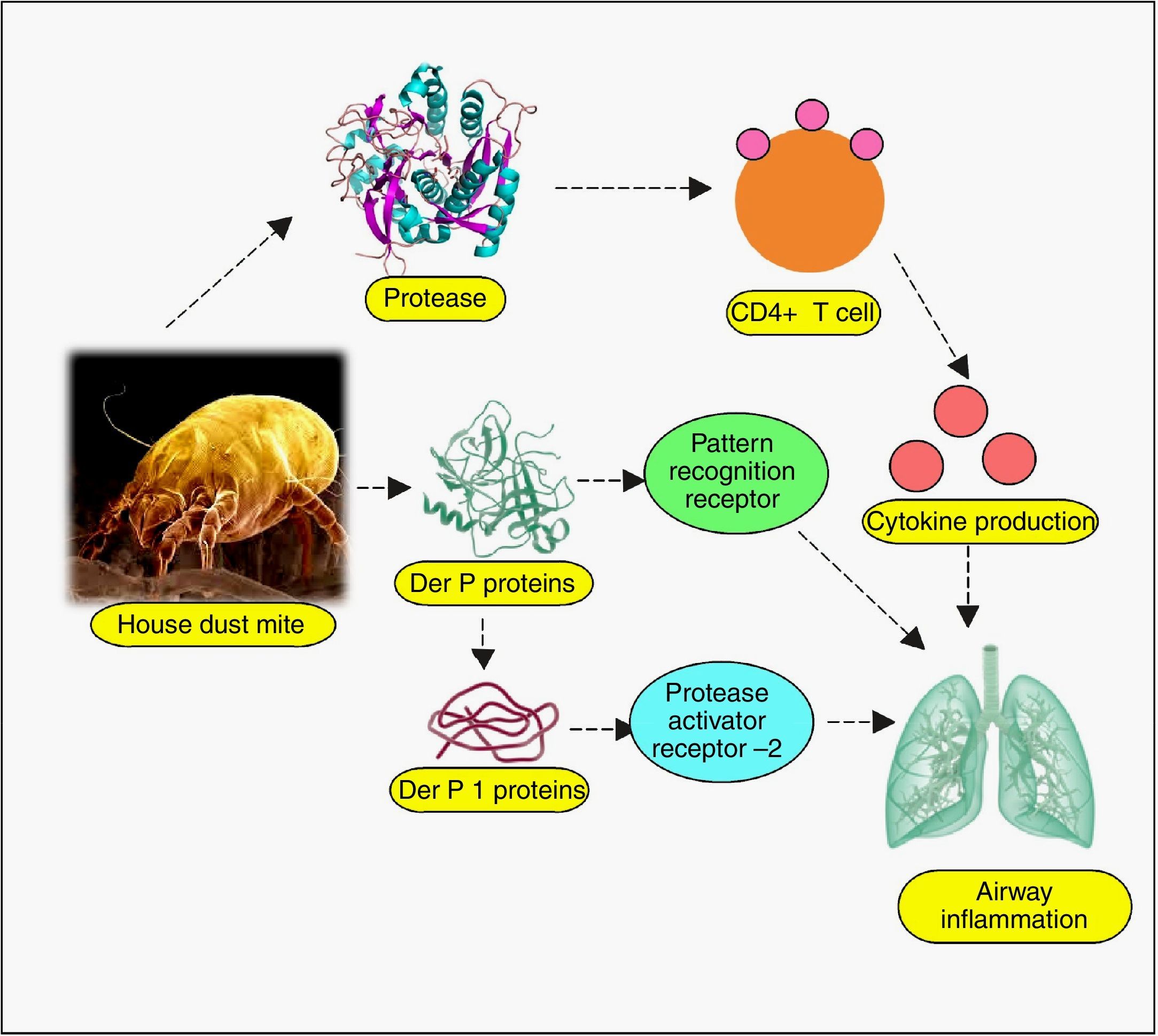
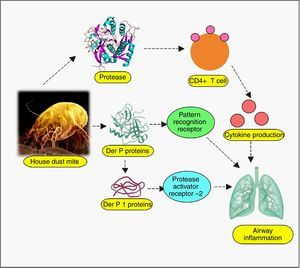
Almost all body parts of the mites, including the gut (oesophagus, proventriculus and other digestive parts), faeces, cuticles and eggs are allergens, triggering allergy in 85% of asthmatics.40 More than 20 house dust mite allergens have been characterised so far, classified into many groups.4 Most of them are proteins, either cysteine proteases belonging to group 1 (papain-like family), or serine proteases belonging to group 2, 3, 6, and 9 (trypsin, chymotrypsin, and collagenase).41 Recently, α-actinin has been identified as a new type of house dust mite allergen.42 Cysteine proteases Der p 1 (from D. pteronyssinus) and Der f 1 (from D. farinae) have been verified to regulate proteolytic activities of all other groups of allergens by zymogen activations, so they have been well-defined.43–45 Evidence suggests that allergenicity can arise from mite-associated bacterial and fungal products as well,40 although they have not been well-explored. Even though explicit allergy elicitation pathways are obscure, the proteases are suspected to be recognised by susceptible individuals’ cell pattern recognition receptors (PRR) such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), retinoic acid-inducible gene 1-like receptors (RLR), NOD-like receptors (NLR) and AIM2-like receptors (ALR), among many other likely players.46–48 These are the same receptors involved in pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs),48 which suggests a connection between infectious and allergic pathogenesis at hitherto esoteric level. Allergen recognition by these receptors, followed by the loss of epithelial integrity and cytokine-driven inflammation cascade manifests as asthma, atopic dermatitis, urticaria, rhinitis, conjunctivitis and other hypersensitivity reactions.49 As for asthma, broncho-constriction followed by pulmonary inflammation has been unravelled.50 Depending on myeloid cell distribution and host of other factors, the allergic responses exhibit themselves. The up-regulated IgE in patient sera, specific to both Der p and Der f proteins, indicates immediate type (Ig E-mediated) allergic reaction.46 Proteolytic activity of these allergens on CD23 and CD25, the peptide chains of cytokine receptors on myeloid cells has been inferred to cause excess IgE synthesis.51 Der p 1 and Der f 1 share extensive sequence similarity, leading to cross-reactivity.34 Der p 2 and Der f 2 are identical by 87%, and they mimic the myeloid differentiation factor 2 (MD-2), the TLR4 complex member, binding to lipopolysaccharide (LPS).52–54
Recent times have unveiled a wealth of information on the pathogenesis of mite allergy, some crucial of which have been analysed below. Epithelial cells are the first line of defence, which undergo perturbation during allergy, especially the cells lining the nasal, airway, pulmonary, mucosal and skin.55 Much investigation has dealt with the permeability upset by allergens and revealed the adverse effects on the tight junctions. Exposure to Der p 1 disintegrates cell-cell adhesion complexes and impairs the expression of transmembrane proteins (occluding, claudin-1 and junction adhesion molecule-A (JAM-A)).56,57 The breakdown of tight junction permits Der p 1 to traverse the epithelial barrier, setting the stage for downstream inflammation. The critical role of tight junctions has been confirmed in that epithelial cells resisted allergens by de novo synthesis of occludins.58 Der p 1 induced inflammatory cytokines (IL-6, IL-8), monocyte chemoattractant protein-1 (MCP-1) and tumour necrosis factor-α (TNF-α) from human monocytic THP-1 cell line.59 Sensitization of this cell line was mediated by β-catenin (a vital protein for intracellular contact and transcription), regulated in turn by Der p 1-phosphorylated glycogen synthase kinase 3β.59 Other studies have also reported that allergic response can be blocked by preventing β-catenin degradation.60 In vitro as well as in vivo studies have revealed that dust mite extract imposes stress on endoplasmic reticulum (ER), mediated via activation of transcription factor 6 α (ATF6α) and protein disulphide isomerase, ERp57.61 Dectin-2, a CLR cluster signalling protein has been found to play a profound role in allergy.62 This protein located on phagocytic cells senses mite allergen and elicits cytokines, arbitrating airway inflammation. To lend further support to this finding, Dectin-2 have been detected in bronchial biopsies of asthma patients63 and dendritic cells.64 The protein on encounter with mite allergen, induced Th2 and Th17 cell differentiation, leading to airway inflammation.64 The comprehensive list of mite allergens has been presented in Table 1.65–67
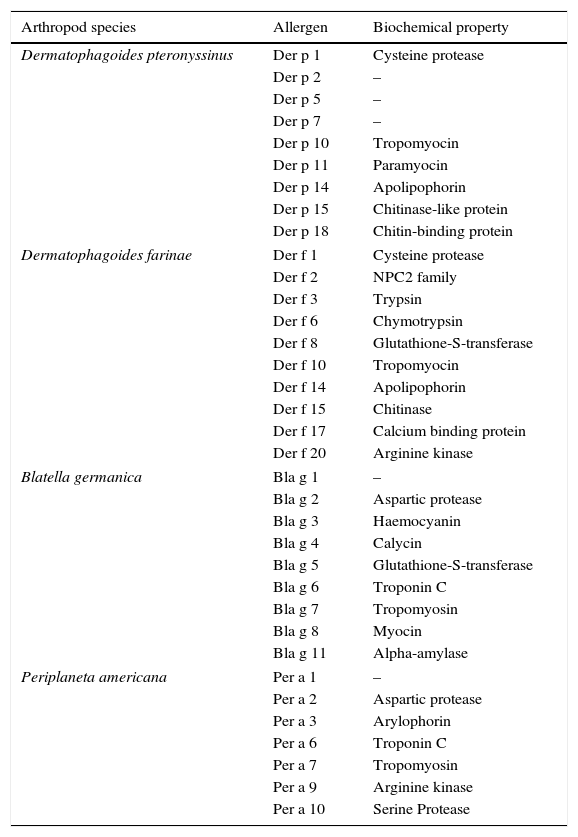
Mites and cockroach species causing allergies, their allergens and biochemical properties.
| Arthropod species | Allergen | Biochemical property |
|---|---|---|
| Dermatophagoides pteronyssinus | Der p 1 | Cysteine protease |
| Der p 2 | – | |
| Der p 5 | – | |
| Der p 7 | – | |
| Der p 10 | Tropomyocin | |
| Der p 11 | Paramyocin | |
| Der p 14 | Apolipophorin | |
| Der p 15 | Chitinase-like protein | |
| Der p 18 | Chitin-binding protein | |
| Dermatophagoides farinae | Der f 1 | Cysteine protease |
| Der f 2 | NPC2 family | |
| Der f 3 | Trypsin | |
| Der f 6 | Chymotrypsin | |
| Der f 8 | Glutathione-S-transferase | |
| Der f 10 | Tropomyocin | |
| Der f 14 | Apolipophorin | |
| Der f 15 | Chitinase | |
| Der f 17 | Calcium binding protein | |
| Der f 20 | Arginine kinase | |
| Blatella germanica | Bla g 1 | – |
| Bla g 2 | Aspartic protease | |
| Bla g 3 | Haemocyanin | |
| Bla g 4 | Calycin | |
| Bla g 5 | Glutathione-S-transferase | |
| Bla g 6 | Troponin C | |
| Bla g 7 | Tropomyosin | |
| Bla g 8 | Myocin | |
| Bla g 11 | Alpha-amylase | |
| Periplaneta americana | Per a 1 | – |
| Per a 2 | Aspartic protease | |
| Per a 3 | Arylophorin | |
| Per a 6 | Troponin C | |
| Per a 7 | Tropomyosin | |
| Per a 9 | Arginine kinase | |
| Per a 10 | Serine Protease | |
Cockroaches have been recognised as one of the most significant allergens.28 Among these insects, two species have been identified to induce most cases of allergies, the German cockroach (Blatella germanica) and the American cockroach (Periplaneta americana).68 Other species such as brown-banded cockroach (Supella longipalpa), brown cockroach (Periplaneta brunnea), Australian cockroach (Periplaneta australasiae), and Harlequin Roach (Neostylopyga rhombifolia) have also been associated with allergies.28 Even the allergenic risk of Madagascar hissing cockroach (Gromphadorhina portentosa), normally kept as a pet, is emerging.69 So, basically all cockroaches are potential allergens, some are well-characterised, some obscure. Among B. germanica allergens Bla g 1, Bla g 2, Bla g 4, Bla g 5, Bla g 6, and among P. americana allergens Per a 1, Per a 3, Per a 7 have been most studied.70 These proteins, characterised as aspartic protease, calycin, troponin, tropomyosin, arylophorin, and glutathione-S-transferase are found in saliva, gut, body extract, faeces, and skin casts of the cockroaches.70 The body extracts of these insects are a cocktail of various proteins, but the most important allergens mediating hypersensitivity have been identified as Bla g 1, Bla g 2 and Per a 1.71 Bla g 1 and Per a 1 belong to group 1, have multiple amino acid residues as tandem repeats, share structural homology (52–72%), are cross-reactive and, both bind to various lipids.71–73 Other significant allergens include Bla g 2 (inactive aspartic proteinase), Bla g 4 (calycin), Bla g 5 (glutathione-S-transferase), Bla g 6 (insect troponin C), Bla g 7 (tropomyosin), Per a 3 (arylphorin), and Per a 7 (tropomyosin),70,74,75 all of them possessing proteolytic properties and variable degree of IgE elicitation responses. Bla g 4 is a male cockroach-specific protein, belonging to the lipocalin (calycin superfamily) family. As is the family characteristic, it binds to small hydrophobic molecules and prompts mammalian immune response.76 Both species of cockroach have pan-allergen tropomyosin, which shares high sequence similarity with other food-grade arthropods, responsible for cross-reactive allergy.77,78 Cockroach allergen-induced allergic inflammation can be both IgE- and non-IgE type, while the former is more common, activation of T cell receptors from various lineages have been recognised as well.79 The host receptors such as protease activated receptor 2 (PAR-2), TLRs and CLRs have been implicated in allergen recognition and subsequent allergy induction.80 Some important host receptors involved in allergy are discussed below. A mice study revealed that mucosal administration of B. germanica faeces protease causes airway inflammation via PAR-2, as the receptor knockout animals had lower airway hyperresponsiveness, and attenuated Th2 and Th17 cytokine level.81 Yet another study explored the role of PAR-2 in mice intranasal sensitisation with cockroach extract, which showed eosinophilic airway inflammation.82 As PAR is a G protein-linked cell surface receptor (GPCRs), mediating signal molecules (hormones, neurotransmitters, and local mediators), its perturbation might be linked to the allergy.83P. americana-sensitisation of human pulmonary epithelial A549 cell line elicited inflammatory cytokine IL-8, with concomitant PAR-2 and PAR-3 mRNA up-regulation, extracellular signal-regulated kinase (ERK/1/2) and Jun N-terminal kinase (JNK) phosphorylation.84B. germanica faeces activated PAR-2, induced chemokine C-C motif ligand 20 (CCL20) and granulocyte macrophage colony-stimulating factor (GM-CSF) production in the airways, increasing the recruitment of dendritic cells to the inflammation site.85 Aryl hydrocarbon receptor, a ligand-activated transcription factor has been implicated in allergy, which modulates the development of specific Th cells.86 In a study, the aryl hydrocarbon receptor sensed the cockroach extract to be an antigen and enhanced the transforming growth factor (TGFβ1) production from fibroblast WI-38 cell line and asthma patient airway epithelial cells. As the link between high TGFβ1 and allergy has been previously established, the role of aryl hydrocarbon receptor in cockroach allergy induction was proved.87 It is noteworthy that many of the discussed receptors are involved in pathogen antigen sensing as well.88 The comprehensive list of allergens has been presented in Table 1.65–67
Remediation, conventional therapies and immunotherapyAbatement of these pests is challenging as the remediation requires integrated approaches.89 For house dust mite elimination, regular washing of the bedding, clothing, furnishing, keeping humidity level below 50%, and using high-efficiency particulate air (HEPA) filter is suggested.90 Pesticide application is required for cockroach management, which in itself carries adverse consequences.91 However, the infestation is a recurring problem as these arthropods persist due to sanitary breaches, geographic and climatic reasons; consequently allergy cases, both acute and chronic cause an immense public health burden.
Like most allergies, the common treatment for mite and cockroach allergy is antihistamines.92 However, it is not a permanent remedy, as the drugs provide mere temporary relief by inhibiting histamine release from mast cells and subsequently lowering inflammation.93 Further, many atopic patients are unresponsive to antihistamines.94 Although corticosteroids (e.g. glucocorticoid prednisolone) constitute another therapeutic regimen, they entail adverse effects as well, in the form of immediate and delayed type hypersensitivity.95,96 Also, corticosteroids can suppress innate immunity by preventing cytokine synthesis, the mediators vital for cell signalling.97 The disruption in cytokine homeostasis leads to metabolic diseases such as osteoporosis, obesity and glucose intolerance, to name a few.97 Further, the compromised immunity favours infection of opportunistic pathogens.98 Overall, the existing drugs are ineffective for restoration of a normal immune state.
As pharmacotherapy has limitations, allergen-specific immunotherapy is considered to be a superior alternative, as it promises long-term treatment, by halting the progression of allergen-induced pathology.99,100 This therapy is based on the administration of the allergens, hence tolerance development towards T cells.101,102 Polymerised allergenic extracts (allergoids) are commonly administered in immunotherapy, to abolish the risk of allergy or anaphylaxis.103 A double-blind, placebo-controlled study was used to track the serum antibody titre in response to the allergoids, which as expected, showed increment in IgG and reduction in IgE level.104 Also, an in vitro study showed that allergoids promote T regulatory cells (Treg) expansion rather than effector T cells activation.103 Polarisation towards Treg, with possibility of reprogramming T cell differentiation implies efficacy of immunotherapy.103,105 In vivo peptide immunotherapy based on allergen-derived epitopes (crucial part of allergen) could be developed against allergic airway inflammation.106 The therapy lowered eosinophilia in the bronchoalveolar lavage (BALF) and ovalbumin-specific IgE, by reducing Th2 cytokines IL-5 and IL-13.106 Subcutaneous immunotherapy was the commonest route of allergoid administration until now, but sublingual immunotherapy is gaining in popularity, due to improved patient compliance.107,108 Despite considerable success in immunotherapy, in the current scenario, it suffers from many hindrances. High sequence diversity of the allergens and heterogeneous makeup of patients make immunotherapy challenging in terms of efficacy.109,110 Massive research on immunotherapy is currently being carried out worldwide, which might in near future, eliminate the hurdles in the path of mass administration.
Crucial domains and emerging frontiersA great deal of work has been done on the biochemical elucidation, tracing the pathological mechanism and therapy against these indoor allergens, yet missing links abound, and guaranteed treatments are still elusive. Further, new discoveries regarding these mechanisms continue to emerge and amend previous fallacies in available information. This review explores the recent strides and emerging paradigms in mite and cockroach allergen research, focusing on budding domains like host immune factors, recombinant technology, role of genetic predisposition in susceptibility, contribution of genome wide analysis (GWAS), metabolomics and beyond. However, apart from the basic mechanisms, several host genotype-specific factors play a role in allergies, which must be recognised.
Host receptors and immune componentsUnderstanding the complexity of the immune system, the interface of innate and adaptive branches, with their myriad ramifications is extremely difficult. However, critical facts are emerging, which can act as a starting point for subsequent discoveries. In this direction, the role of host receptors and Tregs has been discussed below. Dominant allergens of mite and cockroaches are proteases, thus they have the propensity to cleave crucial proteins on epithelial cell surface. Pruritic (itching) role of various proteases as trypsin and tryptase, via GPCRs, especially protease activated receptors (PARs) has been adequately reviewed. Cleaved by serine protease allergen, they undergo activation and expose the system to subsequent immune cell infiltration, nerve and tissue inflammation, causing itching.111,112 In this regard, connexin26, a protein component of gap junction, is pertinent as it coordinates electrical synapses and trafficking of metabolites between adjacent cells, thus restoring integrity of epithelial layer. Certain mutations in gene coding for this protein have been associated with hearing impairment as well.113 A study has unveiled the protective role of connexin26 against Der p 1-caused allergic rhinitis, deficiency of which owing to PAR2 expression makes individuals sensitive to the allergen.114 Allergens imposing stress on endoplasmic reticulum, leading to their dysregulation and protein folding anomaly has been reviewed.115 Impaired protein secretion leads to a dearth of host defence molecules like defensins. In mice model, mite-sensitised epithelial cell endoplasmic reticulum stress has already been associated with higher expression of apoptotic markers, a feature deleterious to the host cells.61 Even the role of parasympathetic nerve signalling on airway smooth muscle and secretory glands, mediating allergic manifestations has surfaced, suggesting cholinergic receptors, especially muscarinic receptors to be of therapeutic importance in allergies.116 The key immunomodulation aspects of allergens have been discussed in other interesting papers, so, instead of repeating it, we would direct the readers to the review on dust mite117 and cockroach allergens80 for detailed information on other significant mechanisms (Figs. 1 and 2).
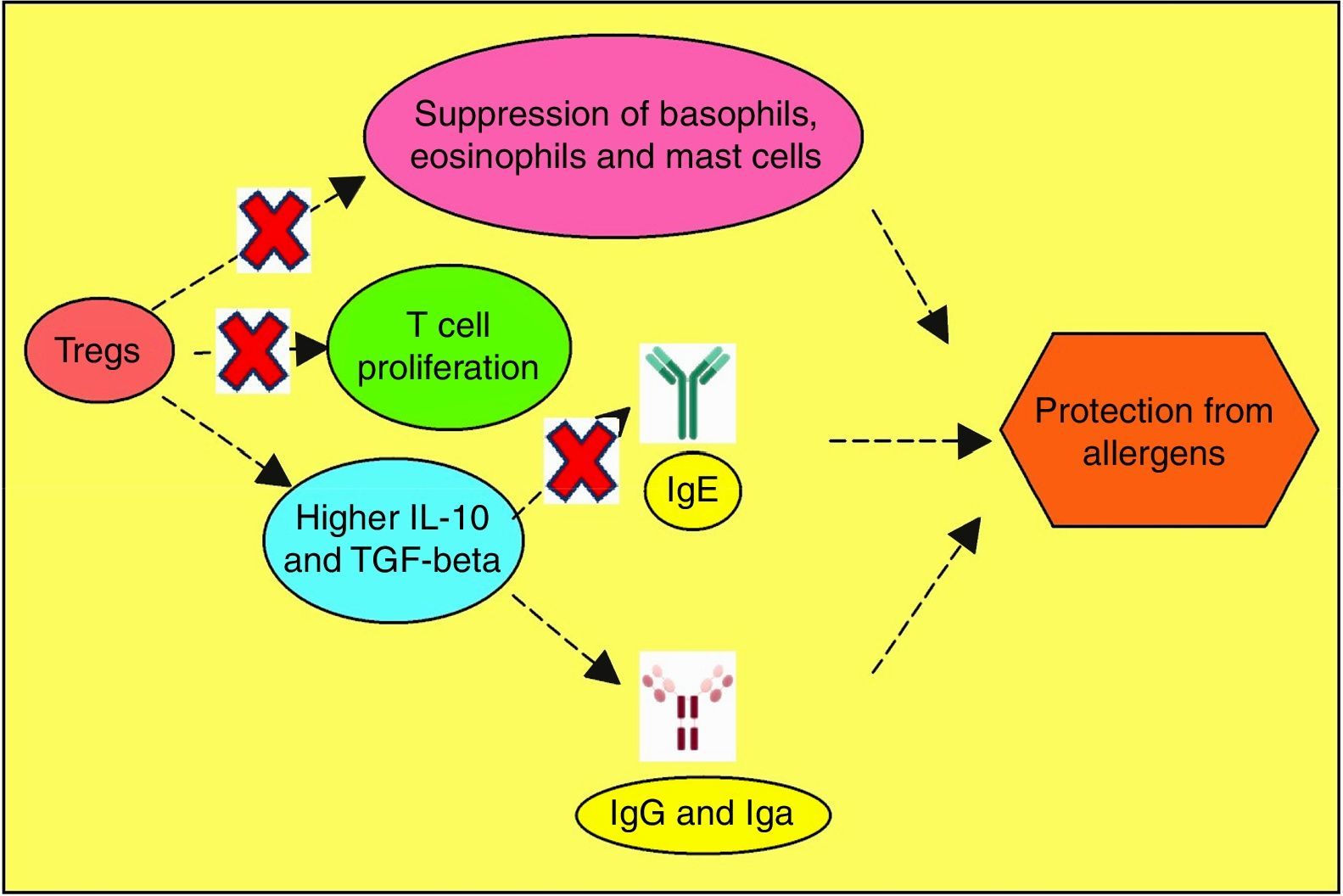
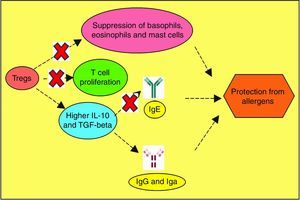
Allergen vulnerability is highly individual-specific, and the repertoire of causal factors is still an enigma. However, two T cell lineages Th2 and Treg have been convincingly implicated in allergies. Tregs (CD4+CD25+ T cells) have been proven to counteract allergies, by dampening T cell proliferation towards Th2 lineage.118 Adoptive transfer of Tregs have been observed to attenuate airway hyperresponsiveness and inflammation.119 Tregs offer protection from inflammatory effects of allergens by multiple mechanisms (Fig. 3). So, impaired Treg permitting allergies was hypothesised and subsequently validated as exemplified below. In allergen-exposed peripheral blood mononuclear cells (PBMCs) from nasal polyposis patients, defective Tregs could not modulate Th1 and Th2 differentiation.120 As Th2 lymphocytes elevate IgE level, promote mast cell rupture and cytokine release, by elaboration of cytokines such as IL-4, IL-5, IL-10 and IL-13, paucity of Tregs are implicated for the predominance of this allergy-favouring phenotype.121 Further investigation towards lineage determination role of Treg revealed the receptor programmed death-1 (PD-1).122 Whether PD-1 is involved in the therapeutic effect of naturally-occurring Tregs (NTregs) and inducible Tregs (iTregs), was evaluated in cockroach allergen-sensitised mice. PD-1, along with its ligands PD-L1 and PD-L2 coordinated the airway hyperresponsiveness, confirming its pivotal role in inflammation. TGFβ regulates Treg maturation and immune homeostasis, so erroneous TGFβ signalling can lead to abnormal Treg functionality.123 Previous discussions have shown the immune polarisation towards Th2 during allergic pathogenesis via cytokines IL-4, IL-5, and IL-13, contributing to airway inflammation and mucus hyper-secretions.124 It needs to be confirmed which signal from dendritic cells drives Th2-dominated T cell differentiation. Apart from the established cytokines, a study found an important role of IL-33, as innate cells responding to this mediator cause airway eosinophilia which the adaptive immunity aggravates (Fig. 4).125
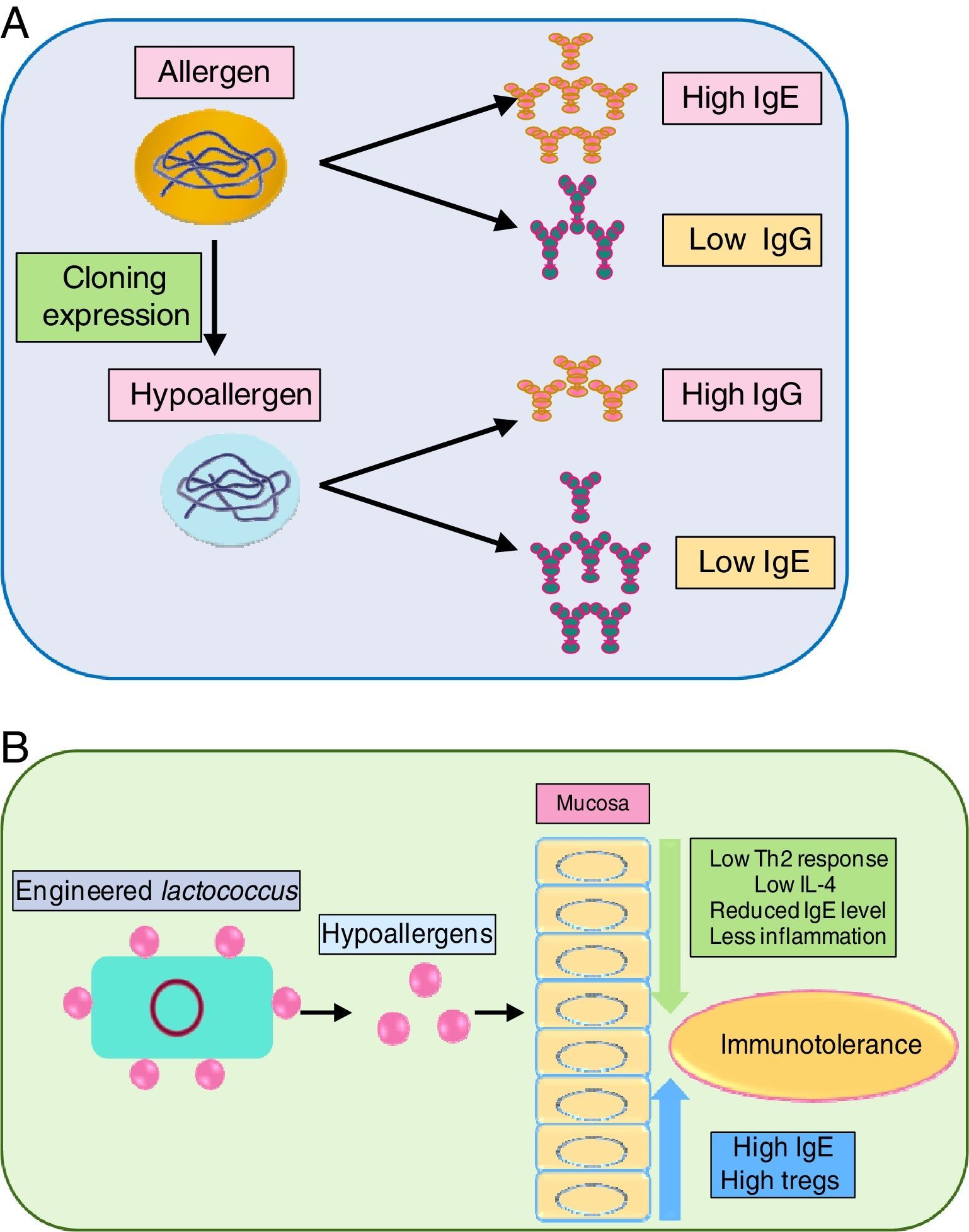
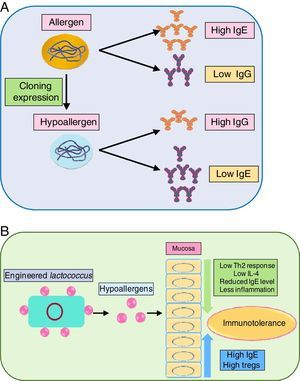
As immunotherapy is the only sustainable way to curb allergens, the development of recombinant allergens with attenuated allergic response (i.e. lower IgE reactivity) is a requisite. Engineered recombinant (by molecular cloning and subsequent expression) allergens possess higher allergy diagnostic power, for skin prick test and IgE quantification. Tempering of the allergens by meddling with their epitope can prove beneficial in immunotherapy. In this regard, a hypoallergenic recombinant allergen rDer p 2/1S against dust mite was developed by expression in E. coli which induced IgG while inhibiting IgE reactivity against Der p 1 and Der p 2 in rabbits.126 Another study also generated hypoallergenic Der p2 with attenuated IgE reactivity.127 Delivery of the recombinant allergen to the inflammation site is envisaged as crucial. In this regard, a recombinant Lactococcus lactis was generated for mucosal delivery of Der p2, which moderated airway inflammation, by lowering Th2 cytokines in BALF via elevating IgG and Treg level while lowering IL-4 level.127
The role of recombinant allergens in cockroach allergy comprehension has been reviewed.71 An in vitro study on P815 mastocytoma cells revealed that recombinant Per a 7 reduces cell surface expression/activation of TLR9 receptors and attenuates IL-12 production. Both the changes occurred by ERK and PI3K/Akt signalling pathways.128 Bla g 7 induced human monocyte-derived dendritic cells to stimulate Th2-specific cytokine production. It indicated a Th2 polarisation.124
Blocking the activity of key allergens is a suitable way to combat allergy. Group 1 allergens of house dust mites, as mentioned before are cysteine protease. In vivo study of house dust mite-challenged animals revealed that targeting just Der p 1 can attenuate severity of allergy.129 It indicated that Der p1 is pivotal in allergenicity and inhibiting it can automatically suppress activity of other dust mite allergens. Immune modulation of recombinant allergens and their delivery via probiotics has been illustrated in Fig. 3(A) and (B), respectively.
Epitope mappingCharacterisation of the allergen loci (epitope) binding to antibody can divulge a lot about the reactive amino acids, sequence conservation with other allergens, cross-reactivity, and safer peptide-based vaccine development,130,131 among others. In this direction, site-directed mutagenesis, microarray immunoassay, enzyme-linked immunosorbent assay (ELISA) and X-ray crystallography have proved very informative. Over the years of epitope mapping, crucial information like amino acid substitutions leading to isoforms and the resultant functional difference among the isoforms have been obtained.132 IgE single-chain variable fragment (scFv) was proposed to be a discrimination marker between Bla g 1 and Per a 1.132 One study located the epitope of Der p2 and modified it, which led to an attenuated allergen, fit for immunotherapy purpose.127 Further elucidation of allergen epitopes can facilitate drug development against them.
Role of analytical techniques and metabolomicsMetabolomics, the emerging omic science appears promising in answering many nagging questions regarding mite and cockroach allergy. Liquid chromatography coupled to mass spectrometry (LC–MS), gas chromatography coupled to mass spectrometry (GC–MS) and proton nuclear magnetic resonance (1H NMR) spectroscopy have enabled the analysis of volatile and non-volatile compounds in metabolome (serum, urine, BALF, serum, and lung tissues) of allergic people. Metabolic changes associated with allergic exacerbations have been detected by these implementations. Biomarker discovery to better understand allergy inducers and mediators have been well-reviewed.133 Metabolomic profiling of urine sample has discriminated between asthmatic and healthy subjects.134 Metabolomic approach to analyse their exhaled breath condensate is being explored. Usage of an integrated GC–MS and LC–MS assay illuminated on metabolic alterations and cytokine changes in metabolome of mite-induced asthma. Eosinophilia, neutrophilia, elevated level of inflammatory cytokines and depleted carbohydrates and lipids (sterols and phosphatidylcholines) were observed.135
Metabolomics techniques have proved their application in structural elucidation of allergens too. Der f 24 (α-actinin) was purified from the dust mite extract, amino acid sequence of which was verified by ESI-QUAD-TOF-based mass spectra analysis.42 Using NMR and isothermal titration calorimetry, it was found that Bla g 4 binds tyramine and octopamine which are adrenergic transmitter analogues of vertebrates. From previous literature, it can be gathered that Bla g 4 uses these amines as ligands.76 Apart from metabolome analysis, these tools can be applied to map epitope of the allergens. A hydrogen/deuterium (H/D) exchange-based-Fourier transform ion cyclotron resonance MS furnished crucial information on almond prunin epitope.136 An informative review on possibility of developing a network by integrating multi-scale data to understand mechanistic of allergies has been published which discusses the key components of systems biology and their analysis.137 This is truly an emerging frontier and the immunological basis of allergy is expected to gain a lot from it.
Human genetic predisposition, sequencing and genome wide analysisThe significance of genetic predisposition in eliciting allergy is a big riddle. Exposed to the same allergen, some individuals develop hypersensitivity, others are unaffected. It substantiates the role of genetic disposition and calls for in-depth investigation of the role of genetics and genome. As the susceptibility to allergies, including dust mite and cockroach, is highly variable from individual to individual, genome analysis might answer the causes to some extent.
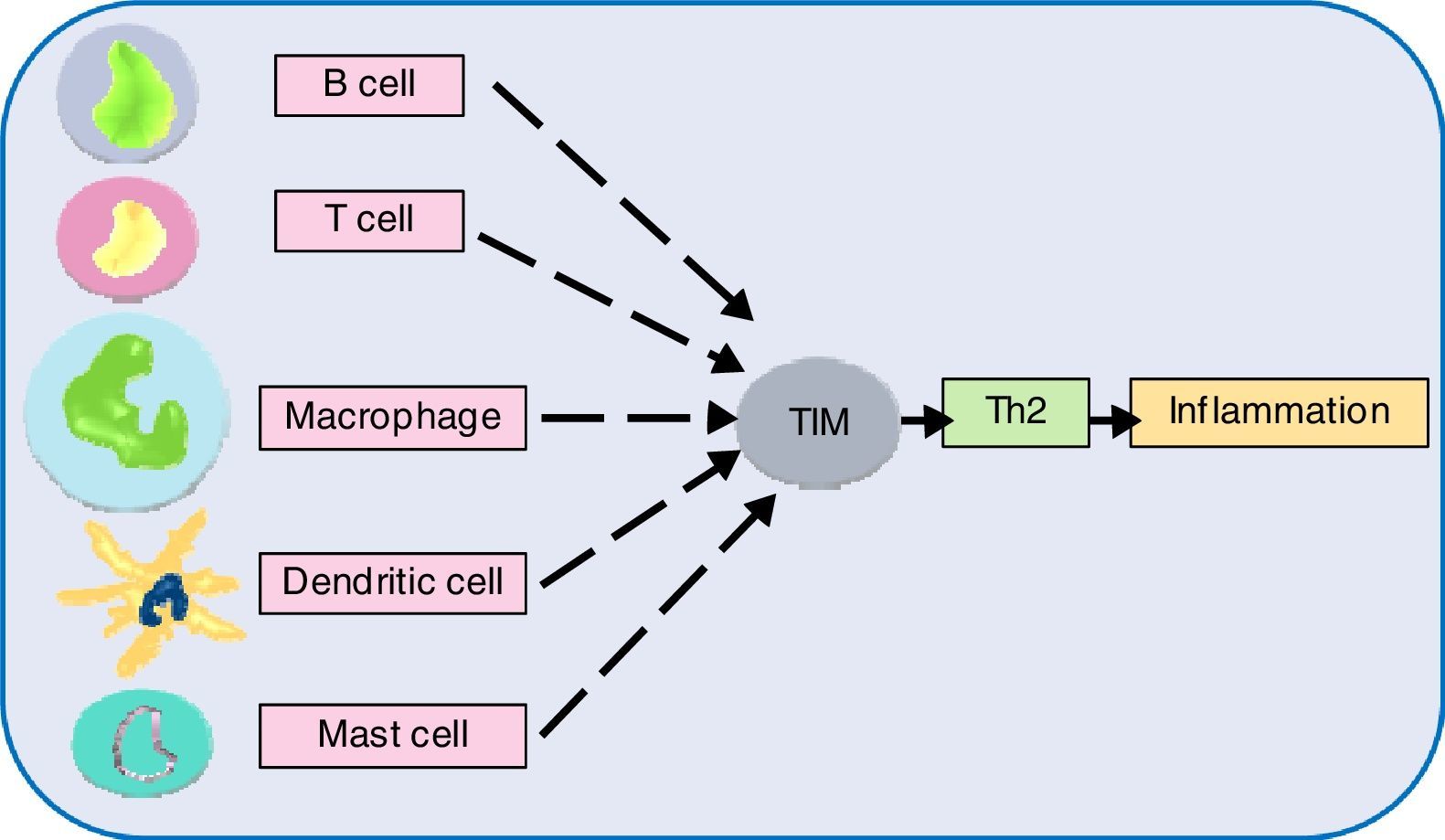
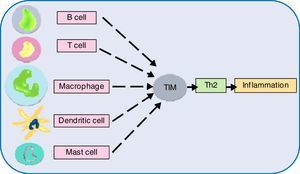
Next generation sequencing (NGS) has taken our understanding of human genetics to the next level. Single nucleotide polymorphisms (SNPs) analyses have facilitated the selection of novel and interesting genes. Investigations in this direction have suggested genetic heterogeneity underlying allergic phenotypes. An overwhelming number of genetic susceptibility loci have been implicated with allergies. The exact number of genes in human is unknown, although the current estimate is about 20,000.138 The genes interact (epistasis) and net result determines expression of a phenotype. Epistasis studies have generated some important findings. Genetic analysis of an allergic rhinitis cohort revealed an epistasis between gene FAM134B and CD39.139 Another study obtained epistatic relation between serine protease inhibitor Kazal-type 5 (SPINK5), and thymic stromal lymphopoietin (TSLP), as a causative factor of asthma.140 Further epistatic interaction determination between genes might divulge crucial pathway information, but such a large set requires the help of robust algorithms.141 In this regard, sequencing followed by genome-wide association study (GWAS) appears promising. This system-wide profiling approach can find genes irrespective of their location, controlling one trait. Sequencing followed by GWAS, epigenetics, transcriptomics might shed light on the underlying molecular mechanisms. Many asthma and atopic dermatitis gene loci and IgE have been identified by GWAS. Genes opposing and favouring allergy risks are inherited in Mendelian inheritance pattern and environmental triggers play a decisive role in the gene expression.142 The review by this author implicates chromosome 5, 6, 11, 12, 16 genes coding for cytokines, MHC IgE, stem cell factors, insulin growth factors, in allergic manifestations. Also, polymorphism of genes for the β-adrenergic receptor, 5-lipoxygenase etc., to name a few have been linked to allergy risks.142 Another gene family T-cell immunoglobulin and mucin domain (Tim) has been discovered to play role in immune perturbations. Tim molecules (namely, Tim 1, 3, 4) have conserved structure and they occur on most immune cell surfaces such as T cells, B cells, dendritic cells, macrophages, and mast cells. Tim members coordinate to promote Th2 response, increasing allergic inflammation.143 Although the precise role of different Tim molecules is not yet clear, investigating them can certainly reveal their role in allergies. GTPase of the immunity-associated protein (GIMAP) family members, have been discovered to play a decisive role in thymocyte development, peripheral lymphocytes apoptosis and Th cell differentiation.144 One study found the role of GIMAP4 and GIMAP5 in allergic sensitisation.145 Deeper study on these small GTPases might contribute to allergic pathologies. A recent study found that an airway epithelial cells protein caspase recruitment domain-containing membrane-associated guanylate kinase protein (CARMA) plays a role in allergies via transcription factor NF-κB activation. In vivo results showed that dendritic cells of animal lacking CARMA3 had compromised antigen processing.146 CARMA family proteins along with other related caspase recruitment domain-containing proteins have been consistently implicated with NF-κB activation followed by signals from cell surface receptors and resultant roles in many diseases.147 Further investigation on these scaffold proteins might illuminate allergic pathways. Another study found the role of allelic variants of tryptase, a secretory product of mast cells. A link between α-tryptase and allergen-specific IgE was observed. A single copy of this tryptase elicited lower IgE, while more copies caused higher IgE level in serum.148 The involvement of tryptases in bronchial hyperresponsiveness triggered by house dust mite allergen might pave the way for further study in this aspect. One study investigated the role of TLR and CD14 substitutions with atopy which led to the finding that certain variants of these receptors offer protection against allergens.149
The gene regions IL33/IL1RL1, IL-13-RAD50 and C11orf30/LRRC32 were associated with multiple allergic phenotypes.150 Many other genes not covered here or hitherto undiscovered genes are likely to be playing critical roles. Connecting the factors and missing links can better unravel this puzzle, which certainly is cumbersome, yet not unattainable.
Other leading frontiersEpigenetics, as the supragenetic modification of chromatin has lately been recognised as a critical factor in mediating diseases and allergies.151 Among the known epigenetic aberrations, DNA methylation, histone modification and microRNA (miRNA) have been studied well.152 A review discusses that epigenomic pattern might indicate the causal stimuli for allergies and enlighten on gene transcription alteration.153 Further, the impact of the epigenetic factors on T cell differentiation and Th2 polarisation has been proposed, which might be causing allergic persistence.152,154 Link between allergy regulatory gene IFNγ promoter methylation and allergic asthma has been established.155 Also, miRNAs, the short, single-stranded RNAs targeting mRNAs have been discovered to interfere with translation. The revised understanding of gene expression has suggested their role in diseases aetiology, including allergies. Precisely, the regulatory role of miRNAs in Th2 polarisation, modification of general inflammatory and tissue responses has come forth.156–158 Among the hundreds of miRNAs, miR-21, miR-146, miR-223, and miR-375 have been associated with allergies.157 It would be interesting to investigate if mite and cockroach allergy are mediated by these RNAs. However, epigenetic studies suffer from unpredictability of heritability or reversible loss, which must be addressed before deriving inferences.
Apolipoprotein E (apoE), a member of the apolipoprotein family of lipid-binding proteins has been found to play a role in lipid homeostasis, hence regulating allergic inflammation.159 Produced by lung macrophages, ApoE has been recognised to render allergic therapy futile by its steroid non-responsive nature.160 When up-regulated, it saturates the low-density lipoprotein receptor (LDLR), triggering airway hyperreactivity.161 In this regard, an apoE mimetic peptide prevented LDLR and resultant inflammation.161,162 This crucial finding might open new avenues for ApoE-imitative drugs discovery, to treat allergic inflammations.
Areas to emphasiseOur exhaustive literature search not only highlighted the seminal findings in recent times, but also reflected several under-appreciated areas. The sections below discuss both aspects, intensely-pursued as well as under-appreciated.
The house dust mite and cockroach allergy was understood to be an outcome of exposure and subsequent challenge to the allergen, thus adaptive immunity is the main player. However, it is not exclusive as genetic predisposition plays a crucial role, highlighting the critical role of innate immunity too. This aspect requires revision and reassessment of previous literature. Correction of pre-established information is as important as discovering new facts, as in the past T cell lineage was thought to be only dichotomous Th1 and Th2, whereas it is much more broader including Tregs/Th3, Th17, Th22, Th9, Th25, NKT etc. The cardinal receptors have been identified as PAR-2, TLRs, CLRs; however there may be numerous others.80
The allergens exert hypersensitivity by enzymatic protease activity, and acting as ligands for various PRRs.
Recombinant allergen engineering using high-level expression vectors has solved many issues inherent to allergen studies. Data have shown their binding ability to serum IgE, thus attenuating mast cell level and inducing eosinophil apoptosis. As a new paradigm of therapy, their application requires to be refined.
Immunotherapy is the only option to nip allergic consequences in the bud. Although it is similar to vaccination against pathogens, departure in functions occurs, which needs to be identified. Peptide immunotherapy has shown IL-10-dependent immunological tolerance by inducing epitope suppression.163
The known arthropod allergens do not appear to account for the full repertoire of IgE responses. A recent investigation identified Der f 24 from D. farinae.164 Understanding will be incomplete until the entire allergen profiles are deciphered. In this regard, metabolomics holds promise and their optimal utilisation should be given thrust. The structural repertoire and epitope mapping can advance cross-reactivity among allergens.
To date, the human genetic makeup was perceived to be homogenous, which the advent of NGS has dismissed. Heterogeneity abounds and the diversity decides allergen susceptibility, unlike the previous allergen-centric assumption. In simple terms, allergen for one person can be perfectly harmless for another person and vice versa. A salient role of genetic predisposition in allergy has been established now, although a gap in knowledge exists. Risk factors need to be mapped. Abnormal T cell proliferation has been found to cause inflammation. Tregs cells capable of correcting them might be defective or absent in predisposed people. HLA-D, TSLP, IL-12A, MBL2 genes have been already established to be involved with cockroach sensitisation.80 GWAS studies of genomics and transcriptomics data might illuminate this area, although knowledge accumulated to date is not very encouraging as manifestations were too different to establish a pattern. The role of HLA leucocyte antigens and other infection (rhinovirus) merits probation. Apart from the genetic and epigenetic factors, other endogenous factors with age and health status compounded with exogenous influences decide the course of allergenicity. Also, the pathologic differences between both gender, owing to hormonal difference, leading to allergies like asthma has come forth.165,166 So, it is a mighty challenge to get rid of allergens. Human systems, despite their stability, are vulnerable to the impact of innumerable intrinsic and extrinsic factors capable of compromising the immune system.
A nexus between allergy and autoimmunity has been proposed before, based on presence of auto-antibodies in the former, just like the latter.167 Link of systemic lupus erythematosus with urticaria and autoimmune thyroid disease with urticaria has come forth.168 Whether allergy creates fertile ground for autoimmunity, or autoimmunity alleles predispose one to allergy requires investigation.
Engineered probiotics are being considered as vehicles of allergoid delivery to gut mucosa as a means of immunotherapy. This area can be pursued for optimal efficacy of immunotherapy.
It is intriguing why some individuals develop respiratory while some suffer dermatological inflammation on challenge with some allergens; while some are unresponsive. The distribution of myeloid cells and inflammasome in human body needs to be characterised. Airway inflammation has already got its fair share of investigation; however, skin immunopathology is rather poorly investigated. Defective epidermal architecture is likely to allow inflammatory mediators for allergic manifestations.
Apart from addressing the above areas, a radical change in our perception to allergies is required, some of house which have been proposed here, which certainly goes beyond dust mite and cockroach allergen. First, the heterogeneity of the human genetic makeup must be appreciated. Second, the biggest loophole in understanding of allergen-induced pathology is that all systems in humans are segregated. In fact, allergy is not only about an agitated immune system, but many more systems, directly or indirectly linked to it. All circuitry crosses paths, yet are delicately balanced; so, perturbation in one path, impacts other allied pathways too. Third, the dominant mite and cockroach allergens are biochemically cysteine protease to which plant protein papain belongs to. Considering many allergens are cross-reactive, it will be interesting to check their crucial polymorphisms and allergic manifestations. For example, the interface of arthropod and food allergy ought to be studied. Fourth, the recent shift from infectious diseases to allergic diseases might have a connection, considering the former is a case of immunodeficiency while the latter is a case of immune-exuberance, which needs to be explored. Further lending support to this antipodal link is that Th1 cells combat intracellular pathogens (mostly immunodeficiency-caused) while Th2 fight extracellular pathogens and command allergic inflammations.
Global warming and the resultant climate change has the potential to favour further proliferation of these allergenic arthropods, which can escalate the magnitude of the current public health problem.169 If not diagnosed and intervened early, the immune system might be provoked, leading to severe consequences. The immune system is one of the most complex of all human systems, its components orchestrating in extreme precision. Tipping the balance in either direction can unleash cascades of pathologies. Given the slow therapeutic progress, despite phenomenal leap in understanding the allergic mechanism, prophylaxis by awareness is the best available option.
ConclusionTo wrap up the topic, allergy is a multifaceted disease with the intricate immune system gone awry. Allergic conditions are on the rise globally, causing a huge health and economic burden. Given their highly personalised nature, unlike other diseases, therapy is not streamlined. Indoor allergens have often been dismissed as trivial health issues, but in the wake of their aggravating role, they are a cause of serious concern. While unprecedented strides in immunology have unveiled the aetiology and therapy to a great extent, missing links exist, comprehension of which for novel therapeutic avenues require research and development in mammoth scale. The above-discussed studies had contributed in that direction in the form of allergen identification, functional elucidation, immunotherapy, recombinant allergen development, epitope mapping, IgE specificity etc. However they are scant, and multi-pronged strategies are requisite to treat them. This review delineating frontline areas like genome-wide analysis studies and metabolomics holds relevance in furthering the understanding of this convoluted topic, by piecing together all these facets and generating startling progress in arthropod-caused indoor allergy.
Funding and ContributorsBRM thanks University Grants Commission (UGC), Govt. of India for the financial support in the form of Dr. DS Kothari Post-Doctoral Fellowship (DSKPDF). The authors would like to that San Diego State University, USA and Indian Institute of Science Bangalore, India for the library access.
Ethical disclosuresPatients’ data protection:
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThere is no conflict of interest in submission of this manuscript.