It is unclear how many children suffering from IgE mediated cow's milk allergy are sensitised to egg in early life and what the clinical implication of this sensitisation is. It is also unclear if those not sensitised to egg in early life, do later on develop sensitisation and clinical allergy to egg.
MethodsThis study examines the prevalence of egg sensitisation among infants with allergy to cow's milk, prior to and following the introduction of egg and what this sensitisation clinically means.
ResultsThe percentage of egg-sensitised children seen among the group of children with cow's milk allergy was 43.2%, and predictive factors for egg sensitisation are discussed. 81.8% of the sensitised patients presented with symptoms when exposed to egg in at least one of its forms, although up to 54.5% of patients tolerated boiled egg and egg-based products. Of the non-sensitised patients, the vast majority (92.5%) did not present with symptoms after the introduction of egg in their diet.
ConclusionsCoexistence of allergy to egg and milk allergy is common, and it is recommended that these patients be monitored, since children who are sensitised to egg despite having never been exposed to it in their diet, may present with symptoms immediately following first ingestion. Most children who are initially non-sensitised to egg do not require special care, and it is not generally recommended to delay or monitor these children, although a small number may have subsequently reacted to egg.
Although limited almost entirely to children, allergy to cow's milk (CM) proteins is one of the most prevalent allergies in our society. A recent review documents prevalence of between 0.3% and 3.5% in young children under five years and less than 1% in older children.1 Previous studies on this subject have revealed that these patients have up to a 50% probability of also developing sensitisation to egg proteins,2,3 even in cases where egg has not yet been introduced into their diet. It is believed that this sensitisation stems from the passage of proteins during breastfeeding; indeed, some studies have reported the presence of egg proteins in breast milk.4 Another possible mechanism behind egg sensitisation is skin damage due to atopic eczema, since patients with atopic dermatitis are often sensitised to egg proteins, whether clinical symptoms exist or not.5 In these infants who are sensitised to egg, symptoms may appear as of the first time the food is consumed, and therefore monitoring of these patients could be recommended,6 although the National Institute of Allergy and Infectious Diseases (NIAID-US) guidelines state that there is insufficient evidence to suggest whether, or which, foods should be tested prior to introduction in children at risk of food allergies (either from a high risk family or with other existing food allergies).7 However, this NIAID statement was not addressed by the World Allergy Organisation (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) guidelines.8
Our aim was to carry out a study to test the following hypotheses: on the one hand, we hypothesised that sensitisation to egg proteins would be higher in the population of patients with CM allergy even where sensitisation was not positively correlated with clinical allergy; and, on the other, that children who are allergic to CM proteins who are not initially sensitised to egg will not present with clinical allergy following the introduction of egg in their diet. To test these hypotheses, we set the following objectives:
- -
to determine the percentage of children with milk-protein allergy who were also sensitised to egg proteins even prior to introduction of egg in their diet;
- -
to determine if clinical or serological differences exist between both groups who are allergic to milk and either sensitised or not sensitised to egg;
- -
to quantify the number (percentage) of egg-sensitised children who are clinically allergic or not allergic;
- -
and, most importantly, to establish the number (percentage) of children not initially sensitised to egg but who later reacted clinically upon the introduction of egg in their diet.
A prospective, analytical, observational study was carried out in the pulmonology–allergology-paediatric outpatient clinic of the Fundación Jiménez Díaz between December 2006 and November 2010 (four years).
Sample size calculationThe proportion required was estimated using the Ene 2.0 program; to obtain a 50% prevalence of sensitisation to egg among the group of patients with allergy to CM proteins, a minimum of 67 patients was required, producing a 95% confidence interval and 12% accuracy.
Inclusion and exclusion criteriaInclusion criteria: children with IgE-mediated allergy to CM initially diagnosed in our outpatient clinic and who, together with IgE determination for milk allergy, had undergone IgE testing for egg and fractions.
Exclusion criteria: children with intolerance or non-IgE mediated allergy; those with prior diagnosis (performed in another centre or previously in the same centre); and, finally, those for whom IgE measurement had not been requested for egg protein along with IgE testing for milk.
VariablesThe variables studied were as follows: sex; age at diagnosis of allergy to CM proteins, presence or absence of pre-existing atopic dermatitis; nearly exclusive breastfeeding or not up to the time of diagnosis; symptoms present at the onset of allergy (cutaneous, gastrointestinal, or anaphylactic); measures of IgE to milk and fractions; and measures of IgE to egg and fractions.
ActionsDiagnosis of allergy to CM proteins was based on patient medical records stating a diagnosis of CM allergy with positive specific IgE testing for CM and fractions (casein, alpha-lactoglobulin and beta-lactoalbumin) (ImmunoCAP, Phadia). Testing of the presence or absence of IgE antibodies to egg and fractions (white, yolk, ovalbumin, and ovomucoid) used the same method.
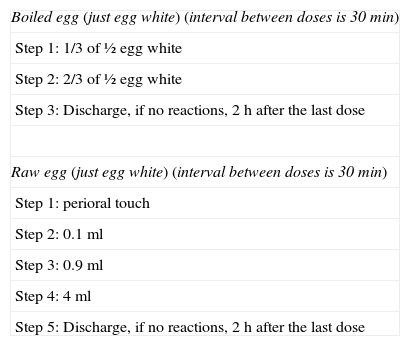
Children with positive IgE results for egg proteins were categorised as allergic to the proteins if IgE to egg white was greater than 6kU/l. This criterion was based on articles demonstrating that the probability of provocation tests being positive is over 95% when values surpass the aforementioned threshold, and therefore testing should be avoided.9 In cases where IgE to egg white was less than 6kU/l – and especially if ovomucoid waskU/l)10 – an open-challenge test was performed using boiled egg (and, at the paediatrician's discretion, raw egg) (Table 1).
Our open-challenge protocol with egg.
| Boiled egg (just egg white) (interval between doses is 30min) |
| Step 1: 1/3 of ½ egg white |
| Step 2: 2/3 of ½ egg white |
| Step 3: Discharge, if no reactions, 2h after the last dose |
| Raw egg (just egg white) (interval between doses is 30min) |
| Step 1: perioral touch |
| Step 2: 0.1ml |
| Step 3: 0.9ml |
| Step 4: 4ml |
| Step 5: Discharge, if no reactions, 2h after the last dose |
We do not usually test the yolk.
For children not initially sensitised to egg, household introduction of egg into the diet was recommended at the customary age (9–12 months), and these patients later underwent follow-up for clinical manifestation of symptoms. In cases where evidence of symptoms appeared, testing of IgE antibodies to egg was repeated. If the presence of symptoms was not established, specific IgE testing was not performed and the patient underwent clinical follow-up exclusively.
Statistical analysisData on the different quantitative variables (IgE) appear as mean figures and 95% confidence interval.
The Kolmogorov–Smirnov test was the first to be used, checking the variables for normal distribution. To compare the quantitative variables obtained for the group of egg-protein sensitised versus non-sensitised patients, Student's (parametric) t test for variables with normal distribution was used and the (non-parametric) Mann–Whitney U test was used for variables that were not normally distributed. Qualitative variables were compared using the chi-squared test.
Statistical analysis was performed with the Statview statistical package (1998).
ResultsDescription of the sampleThe final number of children included in the study was 74. Of these, 32 (43.2%) were previously sensitised to egg despite the food not having been introduced in their diet, while 42 (56.8%) were non-sensitised. Table 2 shows the differences between sensitised and non-sensitised subjects.
Differences, according to the variables analysed, between the group of egg-protein sensitised patients and patients not sensitised to egg proteins.
| Variable | Non-sensitised to eggn=42 | Sensitised to eggn=32 | Statistical significance (Student's t test /X2) |
| Sex | NS | ||
| Male (n%) | 23 (54.8%) | 18 (56.2%) | |
| Female (n%) | 19 (45.2%) | 14 (43.8%) | |
| Age at diagnosis of allergy to CMP (95% CI) | 3.78 months (3.04–4.52) | 4.93 months (4.22–5.64) | P<0.05 |
| Breastfeeding exclusively (%) | 40 (95%) | 30 (93.8%) | NS |
| Prior atopic dermatitis (%) | 15 (35.7%) | 27 (84.4%) | P<0.05 |
| Urticaria at onset (%) | 39 (92.9%) | 29 (90.6%) | NS |
| GI symptoms at onset (%) | 14 (33.3%) | 16 (50%) | NS |
| Anaphylaxis at onset (%) | 2 (4.8%) | 4 (12.5%) | P<0.05 |
| IgE to milk (95% CI) | 7.44 (3.32–11.56) | 18.93 (8.02–29.84) | P<0.05 |
| IgE to alpha-lactoalbumin (95% CI) | 2.44 (0.93–3.95) | 11.43 (4–18.86) | P<0.05 |
| IgE to beta-lactoglobulin (95% CI) | 7.68 (2.54–12.82) | 5.72 (2.31–9.13) | NS |
| IgE to casein (95% CI) | 1.24 (0.71–1.77) | 14.95 (4.46–25.44) | P<0.01 |
A food challenge for boiled egg was performed in children having IgE antibody levels to egg white of <6kU/l, especially in cases where testing for ovomucoid was negative (n=22). Table 3 shows the differences in IgE levels for children with positive challenge tests to boiled egg (clinically allergic) (n=10) (45.4%) and those with negative tests (not clinically allergic) (n=12) (54.5%).
Differences in IgE values for egg proteins between children with negative challenge test (sub-clinical sensitisation) and children with positive challenge test (allergy) (n=22).
| Variable | Not clinically allergic (negative challenge) (n=12) | Clinically allergic (positive challenge) (n=10) | Statistical significance (Student's t test) |
| IgE to egg white (95% CI) | 2.82 (1.53–4.11) | 19.34 (0.87–37.8) | NS (P 0.06) |
| IgE to egg yolk (95% CI) | 0.67 (0.24–1.1) | 4.25 (0.37–8.13) | NS (P 0.05) |
| IgE to ovalbumin (95% CI) | 1.9 (1.16–2.64) | 12.99 (0.17–25.8) | NS (P 0.06) |
| IgE to ovomucoid (95% CI) | 0.57 (0.3–0.84) | 4.63 (0.1–9.37) | NS (P 0.06) |
Of the 12 children who tolerated boiled egg, nine underwent a challenge test with raw egg. Five children did not tolerate raw egg (55.65) despite having tolerated the food when boiled. Only four tolerated egg in its entirety (44.4%), which represents 18.2% of the total group of sensitised patients (81.8% presented with egg allergy in at least one of its forms).
Non-sensitised to eggOf the 42 children not sensitised to egg, just two showed symptoms (urticaria) upon the introduction of the food in their diet (4.8%) and these findings were corroborated by increased IgE levels. One of these two children presented the symptoms when he was first introduced to egg at 10 months, but the other at 18 months, having tolerated egg previously.
None of the 40 remaining patients (95.2%) experienced symptoms following the introduction of egg in their diet.
DiscussionDescription of the sampleOther works appearing in the literature have reported a greater prevalence of sensitisation to egg among patients with allergy to CM proteins.2,3 We have found a similar percentage (43.2%), which means that nearly half of all patients who are allergic to CM are sensitised to egg even if they have not been introduced to egg in their diet.
When analysing the differences between both groups (egg-sensitised and non-egg sensitised) in order to establish criteria for identifying these patients, it is remarkable to see the sensitisation statistics based on patient age, as the group of egg-sensitised patients were slightly older. This fact may be the result of more prolonged breastfeeding and, possibly, delayed introduction of complementary feeding, although this factor has not been analysed and doing so would require specific studies in support of this hypothesis. As reported by other authors,4,5 we have also encountered significant statistical differences in terms of the variable “prior atopic dermatitis”. Based on our study, it is not possible to determine whether atopic dermatitis is a causal factor, with sensitisation occurring through contact between affected skin or, alternatively, if skin condition is merely another risk factor and is not involved in the aetiology and sensitisation, as the process occurs through breastfeeding. Lastly, our findings reached statistical significance in IgE levels, especially to casein, and this may indicate that higher levels of IgE antibodies to milk are correlated with greater probability of sensitisation to egg. It is noteworthy that the level of beta-lactoglobulin is higher in the group of non-sensitised patients, and that this relation nearly reached statistical significance (P=0.56). While further study is necessary to verify this finding, it appears that beta-lactoglobulin may perform a protective role in the process.
Sensitised to eggA controlled oral challenge test was performed in the majority of subjects sensitised to egg using, at least, boiled egg white. The majority of patients sensitised to egg (81.8%) reacted to the raw egg, but only 54.5% reacted to the boiled egg. Other authors have reported lower percentages of positive results in challenge tests performed in sensitised patients (61%).11
Efforts have been made to determine a cut-off point that can be used to predict which patients will have positive results and can therefore be spared unnecessary testing. However, in spite of the fact that IgE values for not clinically allergic patients were apparently lower than for clinically allergic subjects (see Table 3), the levels demonstrating this do not reach statistical significance, probably due to the small sample size and the fact that these levels show a great variability in IgE among clinically allergic children, thus making it difficult to set such a cut-off point. A number of authors have attempted to establish cut-off points regarding skin tests and allergy for both milk and egg.12
Non-sensitised to eggSubjects with no initial sensitisation did not undergo testing following the introduction of egg, and this may be a limitation of the study. Although we do not know if patients tested positively for IgE antibodies, what we do know is what is really relevant: the vast majority of non-sensitised children (95.2%) will not experience clinical problems following the introduction of egg into their diet, meaning that the food can be introduced without limitations or special precaution. We were unable to compare this finding with those of other authors since we did not find any other articles providing this information.
In conclusion, we can say that nearly half the patients with allergy to CM are sensitised to egg. Just a fraction of these will tolerate the food in its whole form, although over half will tolerate it when boiled. From our perspective, the most important finding of this study due to its contribution of new data to the field is that a substantial majority of non-sensitised patients do not experience clinical symptoms following the introduction of the food, thus indicating that special care is not required for these children and that there appears to be no motive for delaying introduction.
Conflict of interestThe authors have no conflict of interest to declare.
We are grateful to Oliver Shaw for his assistance in revising the manuscript in English. We also appreciate Dr. Leandro Soriano assistance in the statistical analysis.