There is accumulated evidence supporting a beneficial role of Mediterranean diet (MD) in the control of asthma symptoms. The aim of this study was to investigate the relationships between adherence to MD and serum levels of certain cytokines namely, interleukin (IL)-4, and IL-17 known to have a pathogenetic role in the airway changes associated with asthma.
MethodsWe measured serum IL-4, IL-33, and IL-17, in 44 asthmatic and 26 healthy children, 5–15 years old. Their adherence to MD was estimated with the Mediterranean Diet Quality Index for children and adolescents (KIDMED) score.
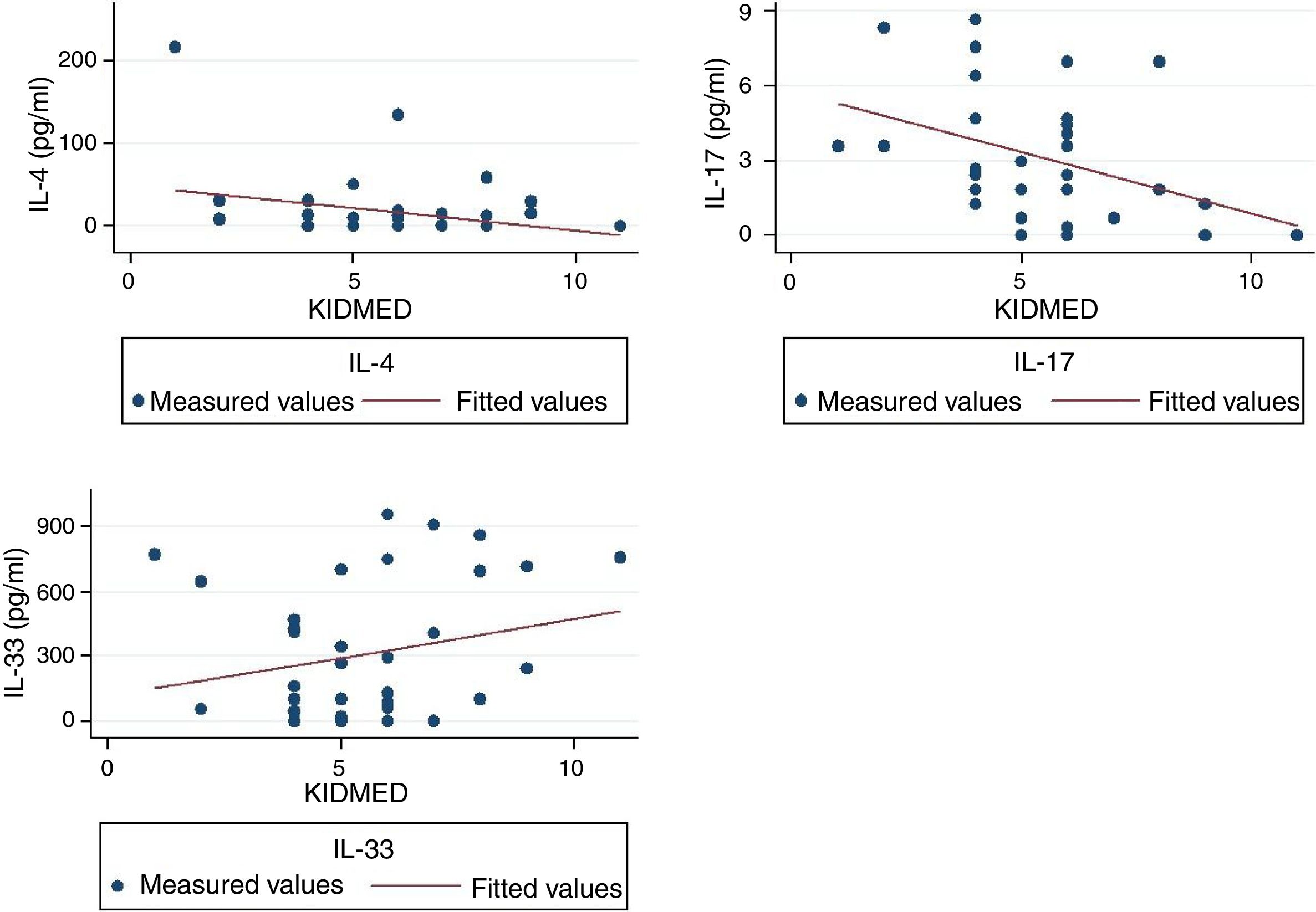
ResultsKIDMED score did not differ between the two groups (P=0.59) and was not correlated with any of the three measured cytokines. However, when the analysis was restricted only to asthmatic children, the KIDMED score was correlated with IL-4, IL-33, and IL-17 (Beta: −0.56, P=0.007; Beta: 0.57, P=0.010; Beta: −0.62, P=0.017, respectively).
ConclusionOur results indicate that MD can modulate the production of some of the main inflammatory mediators of asthma, in asthmatic children.
Asthma is a significant public health problem affecting about 10% of children worldwide. Its pathogenesis is not yet fully elucidated; it is believed to be the result of complex interactions between genetic and environmental factors that predispose to dysfunctional immunological regulatory patterns. Asthma is increasingly considered a syndrome rather than a single disease. During the past few years there has been accumulated evidence supporting the role of diet in the pathogenesis of asthma and much of the research effort has been focused on Mediterranean diet (MD). Indeed, the findings support MD as having a beneficial role in the control of symptoms and maybe in the development of asthma.1–8 MD refers to dietary patterns of populations living in olive-growing areas of the Mediterranean Basin. Despite the different variants of MD, there are certain common components and, in general, MD is characterised by high consumptions of fruits and fresh vegetables, legumes, whole grains, nuts, and olive oil; moderately high intake of fish; moderate intake of dairy products mostly in the form of cheese or yogurt; and low intake of red meat, poultry, sugar and saturated fat.9,10
Asthma involves a complex and extensive crosstalk and interplay between different kinds of cells (mostly T-cells). The exact pathogenetic procedure may be quite distinct for each patient and most probably, it is determined by genetic and environmental factors. The evolving inflammatory process is orchestrated by a cytokine network capable of recruiting and activating a multitude of inflammatory cells in the respiratory tract.11,12
The aim of this study was to delve into the mechanisms through which MD exerts its beneficial effects on asthma, in children. In order to do so, we examined the relationships between adherence to MD and serum levels of certain cytokines namely, interleukin (IL)-4, IL-33 and IL17, known to have a pivotal role in the airway changes associated with asthma.
MethodsTwo groups of children, 5–15 years old, were recruited for the study. The first group (patients) consisted of children diagnosed with intermittent or mild persistent asthma who attended the Children's Respiratory & Allergy Unit of “Attikon” General University Hospital; they had all been treatment naive for at least one month before entering the study. Children in this group underwent a Childhood Asthma Control Test for children (c-ACT) and a score >19 was a prerequisite for entering the study.13 The second group (controls) consisted of healthy children and were enrolled randomly from those visiting the primary care services of our Hospital, either for scheduled vaccinations, or for getting the—mandatory for primary education— pupil's health card. None of these children had been diagnosed with asthma in the past; asthma was further excluded with a thorough history taking.
Children with any kind of chronic inflammatory disease were excluded from the study in order to avoid conditions that could potentially affect cytokines levels. All children were required to be clinically stable for at least four weeks prior to blood sampling.
Venous blood was collected from all participants. Samples were allowed to clot, and serum was separated by centrifuging at 2000g for 10min. Finally, isolated serum samples were transferred to sterile Eppendorf tubes and stored at −80oC for future analysis of IL-4, IL-17 and IL-33. Measurements of serum levels of IL-17 were done with the use of Human IL-17 Quantikine ELISA
Kits (R&D Systems, Minneapolis, MN, USA; Catalog Number: D1700). IL-4 and IL-33 serum levels were measured with Human IL-4 and IL-33 PicoKine ELISA Kits (Boster Biological Technology, Pleasanton CA, USA, Catalog Numbers: EK0404 and EK0929, respectively). The test procedures were performed according to the manufacturers’ directions.
All children underwent complete physical examination, and measurement of body weight and height. Spirometry and skin prick tests to 14 of the most common aeroallergens in Greece were performed on the same day of blood collection.
Adherence to the MD was evaluated with the use of the Mediterranean Diet Quality Index for children and adolescents index (KIDMED).14 The index consists of 16 components that summarise the main characteristics of the MD. The total score ranges from 0 to 12 and is classified into three ordinal categories namely, 8–12, 4–7 and 0–3 for optimal, average and low adherence to MD, respectively.
Written informed consent was obtained from the parents/guardians of all participants. The study was approved by the ethics committee of Attikon Hospital.
Statistical analysisContinuous parameters were expressed as mean±standard deviation (SD). Coefficient of variation (CV) was used to express dispersion of values. Univariate analysis was performed with chi-square, Student's t-test, and Pearson's correlation coefficient (r). Multivariate analysis was performed with three consecutive linear regression models. Each of them had one of the three measured interleukins, namely IL-4, IL-17 and IL-33, as response variable where the two others were used as covariates. KIDMED score, percent predicted forced expiratory volume in one second (FEV1), BMI z-score, allergic sensitisation to any aeroallergen, and presence of asthma, were used as predictive variables. The three models were first applied to the whole study population and then only to the group of asthmatic children. In order to obtain a meaningful comparison between the different regression models we choose to express the results as standardised regression coefficients (Beta).
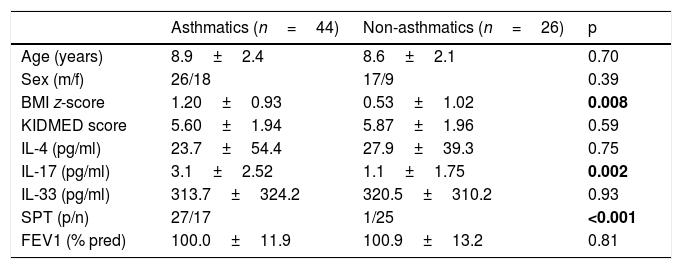
ResultsPatients’ and controls’ clinical characteristics are shown, and compared univariately in Table 1.
Clinical characteristics of patients and controls.
| Asthmatics (n=44) | Non-asthmatics (n=26) | p | |
|---|---|---|---|
| Age (years) | 8.9±2.4 | 8.6±2.1 | 0.70 |
| Sex (m/f) | 26/18 | 17/9 | 0.39 |
| BMI z-score | 1.20±0.93 | 0.53±1.02 | 0.008 |
| KIDMED score | 5.60±1.94 | 5.87±1.96 | 0.59 |
| IL-4 (pg/ml) | 23.7±54.4 | 27.9±39.3 | 0.75 |
| IL-17 (pg/ml) | 3.1±2.52 | 1.1±1.75 | 0.002 |
| IL-33 (pg/ml) | 313.7±324.2 | 320.5±310.2 | 0.93 |
| SPT (p/n) | 27/17 | 1/25 | <0.001 |
| FEV1 (% pred) | 100.0±11.9 | 100.9±13.2 | 0.81 |
SPT: skin prick tests to common aeroallergens; p, positive; n, negative; m, male; f, female.
IL-4 was correlated with IL-33, in the whole study population as well as in the asthmatics group (r=0.51, P<0.001; r=0.53, P=0.003, respectively). No correlation was found between IL-17 and the other two measured cytokines. The mean (SD) KIDMED score of our study population was 5.70 (1.94), and the CV was 34.0%. KIDMED score was not associated with BMI z-score (P=0.20). When we applied the three above-described multivariate models in the whole study populations, the only statistically significant association we identified was that of IL-17 with the presence of asthma (Beta: 0.52, P=0.010). However, when the analysis was restricted to the group of asthmatic children, KIDMED score was found to be correlated with IL-4, IL-33 and IL-17 (Beta: 0.56, P=0.007; Beta: 0.57, P=0.010; Beta: −0.62, P=0.017, respectively). There were no significant correlations between the three interleukins and the rest of the covariates included in the regression models.
The correlations between the three measured cytokines and KIDMED score are shown in Figure 1.
DiscussionIn the present study we evaluated the hypothesis that adherence to MD is related with better regulation of asthma-related interleukins in children. The adherence to MD was assessed with the KIDMED questionnaire, which is considered a quite reliable instrument for this purpose.15 Our results largely corroborated our hypothesis by showing a clear relationship of MD with IL-4, IL-33 and IL-17.
Both groups’ mean BMI z-scores were within normal limits, yet the score was higher in the children with asthma. This observation is consistent with epidemiological findings showing higher BMI among asthmatic children 16; however, it may well be due to a statistical type I error because of our small and not randomly selected sample. The two groups also differed in aeroallergen sensitisation, which was an expected finding given the well-known association between childhood asthma and aeroallergen IgE sensitisation in the school-aged asthmatic children.17 KIDMED was not associated with BMI z-score as adherence to MD does not necessarily imply prevention of adiposity.18 Both groups had, on average, moderate adherence to MD and KIDMED scores were similar between the two groups. Furthermore, the low dispersion of the KIDMED score (as this is indicated by the low CV value) implied relatively uniform dietary habits. This is probably due to the rather homogenous—in ethnic and cultural background—population within the catchment area of our hospital and the adoption of similar dietetic habits.
Literature, undoubtedly, supports the protective role of MD from asthma symptoms.5
Nevertheless, the association of MD with lower prevalence of asthma is less clear.2,19 Although our data did not demonstrate any association of MD with asthma prevalence and are in compliance with those of Barcala et al.19, the small, non-random, convenience sample of our study prevent us from drawing conclusions about this issue.
Serum levels of IL-33 are generally higher in children with asthma and its levels are associated with disease severity.20,21 In the present study, there was no difference between the two groups. A likely explanation is that patients with mild asthma (as is the case in the present study) may have low or even undetectable serum levels of IL-33.20 KIDMED was positively associated with IL-33 in asthmatic children, implying that adherence to MD promotes the production of IL-33. This may come as a surprise since IL-33 is related with disease severity and MD is believed to improve asthma symptoms. However, the role of IL-33 is not univocal. In a recent animal model, it was shown that IL-33-dependent responses of mast cells play a protective and not a causative role in the development of airways hyper-responsiveness.22 IL-33 exerts its actions through its binding to ST-2, which is its receptor on T cells surface. However, ST-2 is not merely bound to the cell membrane but it is also secreted by a variety of cells. When ST-2 is in its soluble form it acts as inhibitor of IL-33 preventing the cytokine's binding to T-cells.12 This may provide another possible explanation for the observed positive association between MD and serum levels of IL-33, in the present study. Unfortunately, we were not able to measure the soluble form of ST-2 to have a more thorough understanding of IL-33 actions in relation to MD.
IL-4 is a major inducer of Th-2 cell development and promotes IgE class-switching in B cells; it also averts the development of type 1 immunity, affecting Th-1 cells and macrophages.12 In our study, serum levels of IL-4 did not differ between the two groups. This is similar to what was observed with IL-33 and—given the strong correlation of the two cytokines—the absence of difference must be attributed to the mild disease and well-controlled symptoms of asthmatic children. The negative association of IL-4 with KIDMED score in asthmatic children implies an inhibiting role of MD on the IL-4 production. A number of animal models have shown that various dietetic interventions can have a protective effect upon allergic airway inflammation reducing, among other things, the levels of IL-4.23–25 However, to the best of our knowledge, this is the first time that such an association is shown in humans.
Increased levels of IL-17 (also called IL-17A) have an important pathogenetic role in patients with severe neutrophilic asthma.26 IL-17 can also promote IL-33-induced airway hyperresponsiveness by enhancing neutrophilic inflammation.27 Furthermore, it has been shown that an unhealthy high-fat diet and the resulting obesity worsen airway inflammation and hyperreactivity through pathways mediated by T-helper 17 cells, and the production of IL-17.28 In a recent study in Puerto Rican children, those on a healthy diet, similar in many respects to MD, had lower levels of interleulin-17F and decreased odds of asthma. The authors were not able to find a statistically significant correlation with IL-17; however, they commented that serum levels of IL17 were markedly lower than those of IL-17F, and so differences were difficult to detect. In our study, IL-17 showed a negative correlation with KIDMED score implying that MD exerts an anti-inflammatory effect by lowering the levels of IL-17 in asthmatic patients.
A main disadvantage of our study was the small sample that obviously decreased the power of our analysis. Another major drawback, also related with the design of our study, is that we did not recruit a random but rather a convenience sample. This may have incurred a degree of selection bias. However, we think that this bias may not have affected markedly our results—at least regarding the comparisons between the two groups—since the main outcomes emerged solely from the analysis of children in the asthma group.
In conclusion, our results indicate that MD can modulate the production of some of the main inflammatory mediators of asthma, and are in accordance with existing epidemiological data that support a beneficial role of MD in controlling symptoms of asthma in children.
Conflict of interestThe authors have no conflict of interest to declare.