A healthy three-year-old boy with no personal or family history of allergic disorders was referred to our allergy department after receiving one dose of hexavalent vaccine (Hexyon®) at age six months. He had presented general discomfort and fever (>37.7°C) for a few days. Fifteen days after vaccination he presented a painful, erythematous indurated skin lesion with edema surrounding the injection site that increasingly took on the appearance of a cutaneous abscess. The lesion resolved with intravenous antibiotics (cloxacillin and clindamycin) and anti-inflammatory treatment (ibuprofen). Surgical drainage of the lesion was also necessary. After receiving a second dose of Hexyon at 18 months, the patient experienced malaise followed by the same cutaneous lesions, although this time with a latency period of seven days and no fever. Once again, it was necessary to administer oral antibiotic therapy (amoxicillin-clavulanic) and anti-inflammatory treatment.
A blood work-up including complete blood count, basic biochemistry, and coagulation study yielded all values within normal limits. The cutaneous abscess was confirmed by ultrasound at the injection site, and the echogenicity observed was compatible with cellulitis. Culture showed the purulent material collected from the drained abscess to be sterile. The patient was subsequently evaluated by the Immunology Department, where a complete study of humoral and cellular immunity was carried out around 10 months after the reactions. The results of the immunological study showed no abnormalities: Ig G 733; IgA 52; IgM 65mg/dl; IgE 8.04IU/ml; anti-tetanus antibodies 31.8mg/l, anti-diphtheria antibodies >3IU/ml. In addition, values for lymphocyte subpopulations were normal (CD3 66%, CD4 41%, CD8 21%, CD19 28%, NK cells 4%) and testing for superoxide anion release by neutrophils in phorbol myristate acetate was positive.
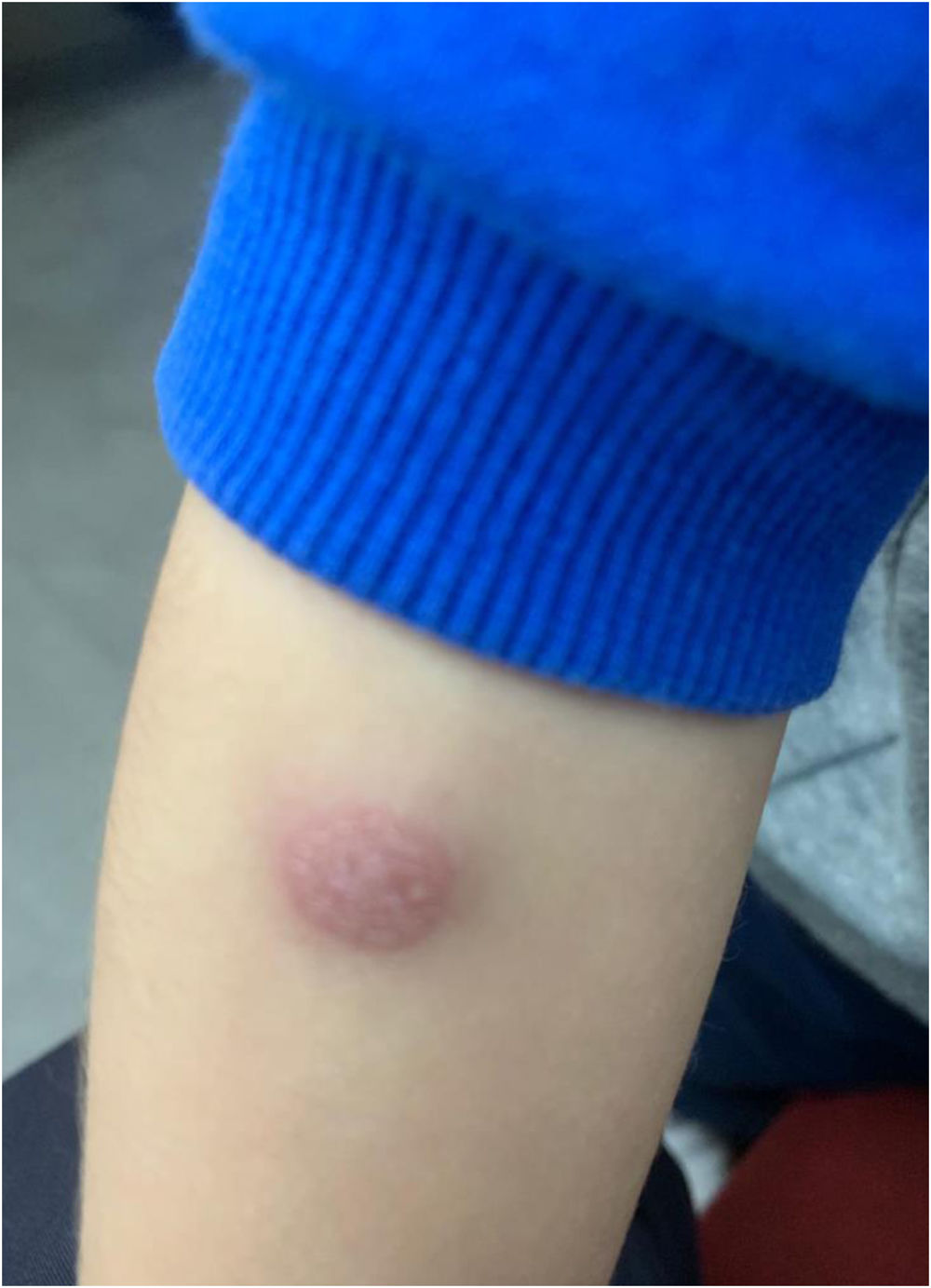
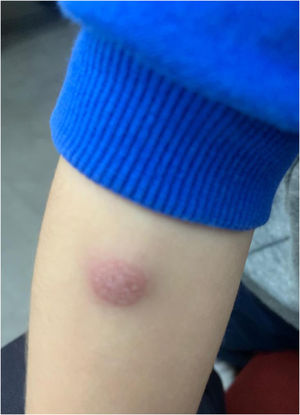
Considering the unusual character of the patient's reaction, it was decided to carry out an allergological study, and informed consent was obtained from the patient's parents. Skin-prick testing with the hexavalent vaccine (Hexyon® lot N1E991V) was performed in duplicate and yielded a negative result. Delayed intradermal skin tests (concentration 1/1) with the vaccine were negative at 20min, although at 48h, a painful, non-pruritic, erythematous induration (4cm×3cm) with an infiltrated central area (1cm×1.5cm) (Fig. 1) had developed in both tests. After five days, a small purulent granuloma appeared at the injection site. This required treatment with corticosteroids and topical antibiotics (fusidic acid and betamethasone). Patch tests were performed with the same hexavalent vaccine in duplicate (undiluted), with a positive result at 48 and 96h.
Patch testing with a standard battery including neomycin (True Test®) was negative. Given the possible implication of a cellular immune mechanism, we decided to carry out a lymphocyte transformation test (LTT) with Hexyon® vaccine and with the excipients (neomycin and streptomycin). The result was negative with Hexyon® and streptomycin and positive with neomycin (stimulation index at a concentration of 100μg/mL, 2.3).
Due to the patient's age, it was necessary to administer the vaccines corresponding to varicella and measles/rubella/mumps, which also contain neomycin and other agents (disodium phosphate, potassium phosphate, sucrose and hydrochloric acid) as excipients. Skin tests (prick test and intradermal-test; concentration 1/1) were performed with the two vaccines (Varivax® and M-M-M vaxPro®). Immediate and delayed readings were negative. Subsequent administration of both vaccines was well tolerated.
Several months later, the patient had to be vaccinated against meningococcus with “Bexsero®” (GlaxoSmithKline), which contains 0.5mg of aluminum hydroxide (A13+) as an excipient. Before administration, skin tests (prick test and intradermal test; concentration 1/1) were performed with the vaccine. The immediate reading at 20min and subsequent readings, at 48h and seven days were negative. The first dose of Bexsero® was well tolerated. No complications were observed after administration of a second dose six months after.
While vaccinations play an important role in the prevention of infectious diseases for society as a whole, it is important to recognize the many cutaneous reactions induced by vaccines. Hence the importance of the present case: un unusual reaction to a vaccination in a pediatric immunization schedule. Adverse reactions to vaccines may be directly related to the vaccine or the adjuvant or may be due to non-specific inflammation at the injection site.1 The Hexyon® vaccine, (Sanofi-Pasteur-MSD), a hexavalent vaccine (DTaP-IPV-HB-Hib), is indicated for primary and booster vaccinations against diphtheria, tetanus, pertussis (acellular component), hepatitis B (rDNA), polio (inactivated), and Haemophilus influenzae type-b conjugate in infants and children from six weeks of age. It may contain traces of glutaraldehyde, formaldehyde, neomycin, streptomycin, and polymyxin B, which are used during the manufacturing process. As with many other vaccines, hexavalent vaccine has a good safety and immunogenicity profile.2,3
In children, the most frequent adverse reactions to vaccines are injection site pain and erythema, irritability, and crying. In some cases, the reaction may be significant and may extend beyond the injection site for several days.4 These unusual local reactions arise because of inadequate administration technique (subcutaneous vs. intramuscular) and the presence of adjuvants such as aluminum hydroxide, formaldehyde, and glutaraldehyde. Type III hypersensitivity reaction may also be triggered by the production of immunocomplexes that cause reactions such as Arthus phenomenon.5
Given that excipients were excluded as causal agents, the reaction in the present case may have been the result of an Arthus phenomenon or another unusual reaction to a vaccine, such as erythema nodosum.6,7 Therefore, we measured the anti-tetanus and diphtheria antibody concentrations, as very high titers could be indicative of Arthus phenomenon. However, the result was normal in the present case. It was not possible to determine specific antibody levels for the other components of the vaccine (pertussis, hepatitis B, polio, and Haemophilus Influenzae type-b). In these cases, we should consider whether to continue administering vaccines with lower antigen content or to avoid their administration altogether. In the present case, this issue was not considered, since the patient did not need further doses of the vaccine.
We could not demonstrate the implication of a cellular mechanism despite performing the LTT. Although a positive result was obtained in the LTT with neomycin, the negative patch-test result with neomycin and the subsequent tolerance of additional vaccines containing this antibiotic rule out its possible implication. Aluminum may also have been the causal agent,8,9 although the fact that the patient tolerated a vaccine (Bexsero®) with a similar concentration of aluminum to that of Hexyon® vaccine suggests that this agent was not involved.
A large variety of autoimmune reactions are thought to be triggered by vaccines, although, the evidence for such associations remains largely anecdotal. Our literature search did not reveal similar cases of adverse reactions to this vaccine, other than those already mentioned.2,4 It is important to identify these reactions in order to manage them correctly. In addition, it is necessary to educate patients on the critical importance of vaccinations, which are key public health tools for the control of immunopreventable diseases.