Aeroallergens are airborne organic substances which are responsible for allergenic diseases in hypersensitive individuals. People are exposed to their allergens either directly or after their entrance into the interiors. The spatio-temporal pattern of aeroallergens and their relationship with weather variability in Abuja and Nassarawa, North-Central Nigeria was studied.
Materials and MethodsAerosamples were trapped with modified Tauber-like pollen traps. Samples were collected monthly and centrifuged at 2500rpm for 5 min and subjected to acetolysis. Meteorological data were collected from the Nigerian Meteorological Agency.
Results and ConclusionAeroallergens concentration were unequivocally regulated by weather variables in both locations, indicating the possible use of aeroallergens especially pollen and spores as bio-indicators of weather variations and change. Aeroallergens encountered were fungal spores, pollen, diatom frustules, fern spores, algal cyst/cells in decreasing order of dominance. Among pollen group, Poaceae, Amarathaceae/Chenopodiaceae and Hymenocardia acida dominated. Spores of Smut species, Puccinia, Curvularia and Nigrospora were major contributors among aeromycoflora. Fungal spores morphotype dominated during the rainier months and were major contributors of the aeroallergen spectrum with those belonging to Deuteromycete preponderant. Aeroallergens which were previously identified as triggers of conjunctivitis, asthma, allergic sinusitis and bronchopulmonary allergic diseases were frequently present in both locations. Pollen prevailed more during the harmattan, influenced by northeast trade wind. Pollen component differed and was based on autochthonous source plants, indicating difference in sub-vegetational types.
Aeroallergens are continuous component of the air, and their type and abundance vary according to biological and environmental factors1. Airborne pollen and spores are the most dominant aeroallergens, other types include but not limited to diatom, algal cysts, and insect parts. Most are responsible for allergic asthma, conjunctivitis, rhinitis/hay fever, etc. Spores of fungi play a crucial role in allergy and also in agriculture because of their involvement in plant diseases epidemics.
Pollen grains as aeroallergens are well documented across the world and are important causes of pollinosis1. Pollen dispersed from grasses, weeds and trees are major sources of allergens and show characteristics of seasonal pattern of occurrence2. Pollen allergens are considered a major risk factor for both seasonal allergic rhinitis and asthma, whereas indoor allergens appear to be a risk factor for perennial rhinitis3. The release of allergenic particles from the pollen cytoplasm is accentuated by isotonic and hypotonic medium which enable them to diffuse on the mucosa surfaces such as the conjunctiva and the nose4.
Fungal spores are always a component of the atmosphere, constituting the largest proportion of aerobiological particles in our environment, which even exceeds the concentration of pollen grains but are much less studied than them1,5. Fungi cause toxic and allergic reactions, toxic reactions are non-immunological and occur with first encounter, allergic reaction on the other hand requires previous sensitizing exposure6. Numerous species of fungi have been discovered as triggers of allergy although their contributions are species-specific. The significance of genera from Alternaria, Cladosporium, Penicillium, Aspergillus, Curvularia, and Malassezia has been documented7. Sensitization to Alternaria alternate has been linked to a major risk factor for fatal asthma attacks8.
The incidence of fern spores among aerospores could suggest the possibility of them having an adverse effect on human beings after inhalation or deposition on skin 9. However, there is dearth of knowledge on fern spores allergenicity. Many aeropalynological studies in Nigeria recorded lots of fern spores in the atmosphere.10,11 There is evidence of allergenic material in both sporangial matter and spores of Acrostichum aureum and it displayed a positive response in patients.9,12
Allergenicity due to airspora is usually not life threatening but can severely affect patients’ quality of life. Unlike food allergy, aeroallergens can hardly be avoided because of their extremely small sizes. Studies on spatio-temporal pattern of aeroallergens are important in discovering their risk period of occurrence in ambient air, this knowledge is imperative in prophylaxis and immunotherapy. There are few published articles on aeroallergens in Northern Nigeria.11,13,14 The present research is the first aeropalynological work in Nassarawa and also the first to compare impact of climate variables on aeroallergen spatio-temporal distribution in North-Central Nigeria.
Materials and methodsStudy areaAbuja and Nassarawa states are contiguously located in North-Central Nigeria. The former is the Federal Capital Territory of Nigeria, made up of six local councils, comprising the City of Abuja. and five Local Government Areas. Nassarawa is made up of 13 local government Areas. Abuja and Nassarawa lie within latitude 8° 25’N and 8° 32N and longitude 7° 451 E and 8° 181E and occupy a land area of about 27,117km2 and 7314.2km2, respectively11,15,16. Both fall within the guinea Savanna vegetation zone of Nigeria and experience wet season and dry season, part of dry season (harmattan) is characterized by northeast trade wind with main features of dust haze, cold and dryness.17 In Abuja, vegetation is made up of patches of lowland rainforest taxa and savanna woodland species.17 Vegetation in Nassarawa is sparser than that in Abuja. There is little dense forest which is found in lowland areas particularly where population pressure is less on land.18 The study locations were in Garki and Karu in Abuja (FCT) and Nassarawa State respectively. The selected study locations fulfilled three important criteria: accessibility, free flow of air and free from vandalization.
Sampling was carried out with Tauber-like pollen traps modified to 1.52m above ground level. A concoction of chemicals consisting of glycerol (50mL), formaldehyde (10mL) and phenol (5mL) were prepared and poured into the traps, which were mounted on the first day of the month and replaced after each monthly recipient collection. Collected recipients were stored in a refrigerator to avoid any ongoing oxidation of plants materials. Sampling was carried out for six months, from January to June, 2017. Prior to laboratory analysis, collected samples were sieved using 100μm mesh wire gauze and centrifuged at 2500rpm for 5min. Sediments were subjected to acetolysis following an established procedure11.
Meteorological data: rainfall (mm), temperature (oC), wind (knots), humidity (%) which span from January to June, 2017 were collected from the Nigerian Meteorological Agency.
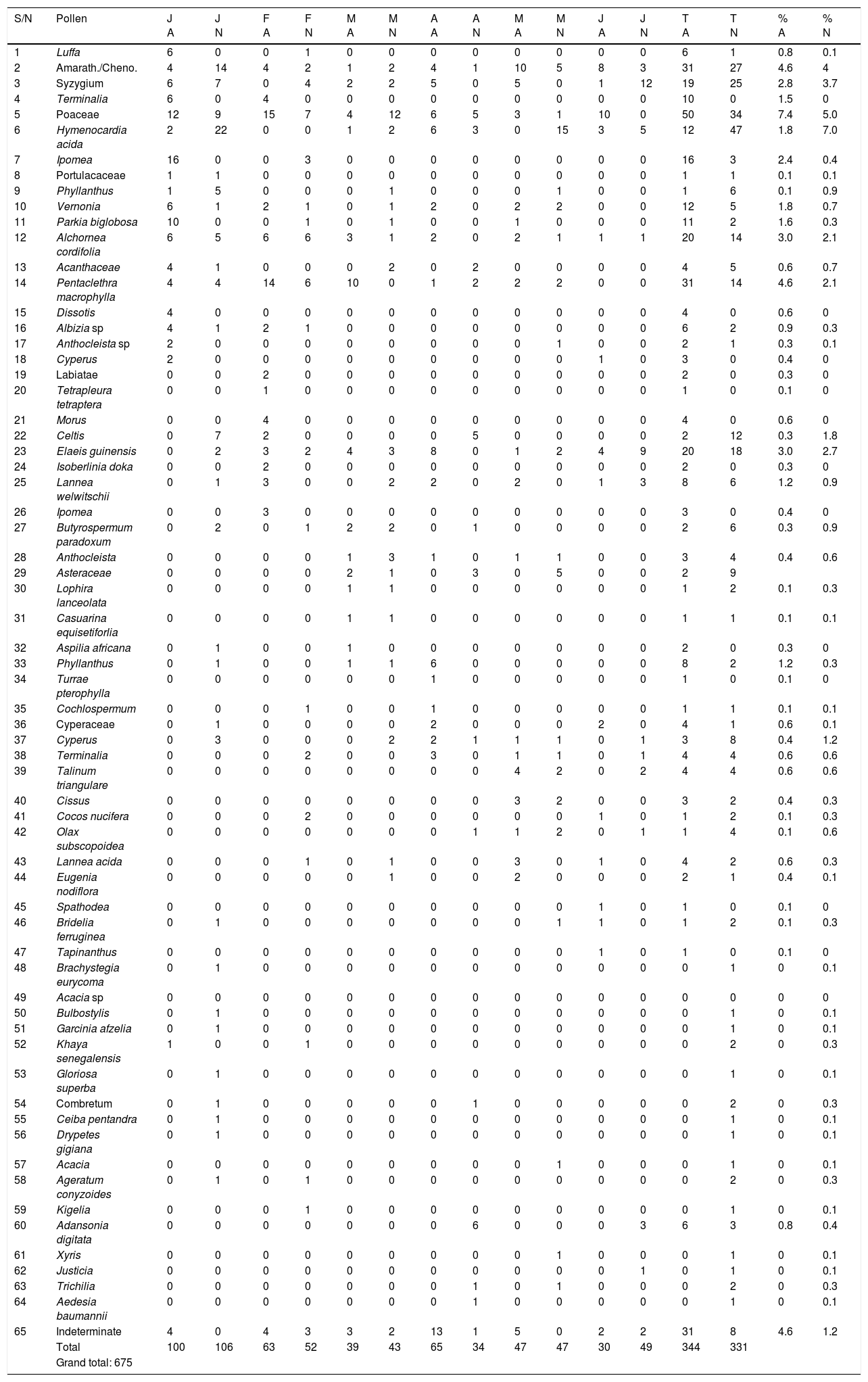
ResultsAeroallergens spatio-temporal pattern in Abuja and Nassarawa, North-Central NigeriaPollenA total of 675 pollen comprising 64 types and indeterminate were recorded in both locations. Nassarawa had a record of 52 pollen types, whereas Abuja achieved 50 types. Most pollen were dispersed from enthomophilous taxa. Pollen dispersed from Poaceae, Pentaclethra macrophylla, Elaeis guineensis, Alchornea cordiforlia and Amarathaceae/Chenopodiaceae dominated in Abuja, whereas Syzygium, Hymenocardia acida, Poaceae and Amaranthaceae/Chenopodiaceae pollen were more abundant in Nassarawa. Peculiar to Nassarawa were pollen released from Brachystegia eurycoma, Acacia, Ceiba pentandra, Eugenia nodiflora, Gloriosa superba, etc. In both locations pollen were more preponderant in quantity and morphotypes in December. In Abuja, pollen declined progressively from January 100 (14.8%) through February 63 (9.3%) to March 39 (5.8%), appreciated in April 65 (9.6%) and declined in May 47 (7.0%) and June 30 (4.4%). In Nasarawa, pollen declined after a higher input in January 106 (15.7%) through February 52 (7.7%), March 43 (6.4%), April 34 (5.0) and increased again in May 47(7.0) and June 49 (7.3%) (Table 1).
Monthly pollen count (per 100μL of acetolyzed sample) in aerosamples from study locations.
| S/N | Pollen | J A | J N | F A | F N | M A | M N | A A | A N | M A | M N | J A | J N | T A | T N | % A | % N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Luffa | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 0.8 | 0.1 |
| 2 | Amarath./Cheno. | 4 | 14 | 4 | 2 | 1 | 2 | 4 | 1 | 10 | 5 | 8 | 3 | 31 | 27 | 4.6 | 4 |
| 3 | Syzygium | 6 | 7 | 0 | 4 | 2 | 2 | 5 | 0 | 5 | 0 | 1 | 12 | 19 | 25 | 2.8 | 3.7 |
| 4 | Terminalia | 6 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 1.5 | 0 |
| 5 | Poaceae | 12 | 9 | 15 | 7 | 4 | 12 | 6 | 5 | 3 | 1 | 10 | 0 | 50 | 34 | 7.4 | 5.0 |
| 6 | Hymenocardia acida | 2 | 22 | 0 | 0 | 1 | 2 | 6 | 3 | 0 | 15 | 3 | 5 | 12 | 47 | 1.8 | 7.0 |
| 7 | Ipomea | 16 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 3 | 2.4 | 0.4 |
| 8 | Portulacaceae | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.1 | 0.1 |
| 9 | Phyllanthus | 1 | 5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 6 | 0.1 | 0.9 |
| 10 | Vernonia | 6 | 1 | 2 | 1 | 0 | 1 | 2 | 0 | 2 | 2 | 0 | 0 | 12 | 5 | 1.8 | 0.7 |
| 11 | Parkia biglobosa | 10 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 11 | 2 | 1.6 | 0.3 |
| 12 | Alchornea cordifolia | 6 | 5 | 6 | 6 | 3 | 1 | 2 | 0 | 2 | 1 | 1 | 1 | 20 | 14 | 3.0 | 2.1 |
| 13 | Acanthaceae | 4 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 4 | 5 | 0.6 | 0.7 |
| 14 | Pentaclethra macrophylla | 4 | 4 | 14 | 6 | 10 | 0 | 1 | 2 | 2 | 2 | 0 | 0 | 31 | 14 | 4.6 | 2.1 |
| 15 | Dissotis | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0.6 | 0 |
| 16 | Albizia sp | 4 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2 | 0.9 | 0.3 |
| 17 | Anthocleista sp | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0.3 | 0.1 |
| 18 | Cyperus | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0.4 | 0 |
| 19 | Labiatae | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.3 | 0 |
| 20 | Tetrapleura tetraptera | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 | 0 |
| 21 | Morus | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0.6 | 0 |
| 22 | Celtis | 0 | 7 | 2 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 2 | 12 | 0.3 | 1.8 |
| 23 | Elaeis guinensis | 0 | 2 | 3 | 2 | 4 | 3 | 8 | 0 | 1 | 2 | 4 | 9 | 20 | 18 | 3.0 | 2.7 |
| 24 | Isoberlinia doka | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.3 | 0 |
| 25 | Lannea welwitschii | 0 | 1 | 3 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 1 | 3 | 8 | 6 | 1.2 | 0.9 |
| 26 | Ipomea | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0.4 | 0 |
| 27 | Butyrospermum paradoxum | 0 | 2 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 6 | 0.3 | 0.9 |
| 28 | Anthocleista | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | 4 | 0.4 | 0.6 |
| 29 | Asteraceae | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 3 | 0 | 5 | 0 | 0 | 2 | 9 | ||
| 30 | Lophira lanceolata | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0.1 | 0.3 |
| 31 | Casuarina equisetiforlia | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.1 | 0.1 |
| 32 | Aspilia africana | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.3 | 0 |
| 33 | Phyllanthus | 0 | 1 | 0 | 0 | 1 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 8 | 2 | 1.2 | 0.3 |
| 34 | Turrae pterophylla | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 | 0 |
| 35 | Cochlospermum | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.1 | 0.1 |
| 36 | Cyperaceae | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 4 | 1 | 0.6 | 0.1 |
| 37 | Cyperus | 0 | 3 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 1 | 0 | 1 | 3 | 8 | 0.4 | 1.2 |
| 38 | Terminalia | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 1 | 1 | 0 | 1 | 4 | 4 | 0.6 | 0.6 |
| 39 | Talinum triangulare | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 2 | 4 | 4 | 0.6 | 0.6 |
| 40 | Cissus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 3 | 2 | 0.4 | 0.3 |
| 41 | Cocos nucifera | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0.1 | 0.3 |
| 42 | Olax subscopoidea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 4 | 0.1 | 0.6 |
| 43 | Lannea acida | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 4 | 2 | 0.6 | 0.3 |
| 44 | Eugenia nodiflora | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0.4 | 0.1 |
| 45 | Spathodea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0.1 | 0 |
| 46 | Bridelia ferruginea | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0.1 | 0.3 |
| 47 | Tapinanthus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0.1 | 0 |
| 48 | Brachystegia eurycoma | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 49 | Acacia sp | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 | Bulbostylis | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 51 | Garcinia afzelia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 52 | Khaya senegalensis | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.3 |
| 53 | Gloriosa superba | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 54 | Combretum | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.3 |
| 55 | Ceiba pentandra | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 56 | Drypetes gigiana | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 57 | Acacia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 58 | Ageratum conyzoides | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.3 |
| 59 | Kigelia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 60 | Adansonia digitata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 3 | 6 | 3 | 0.8 | 0.4 |
| 61 | Xyris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 62 | Justicia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0.1 |
| 63 | Trichilia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0.3 |
| 64 | Aedesia baumannii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 65 | Indeterminate | 4 | 0 | 4 | 3 | 3 | 2 | 13 | 1 | 5 | 0 | 2 | 2 | 31 | 8 | 4.6 | 1.2 |
| Total | 100 | 106 | 63 | 52 | 39 | 43 | 65 | 34 | 47 | 47 | 30 | 49 | 344 | 331 | |||
| Grand total: 675 |
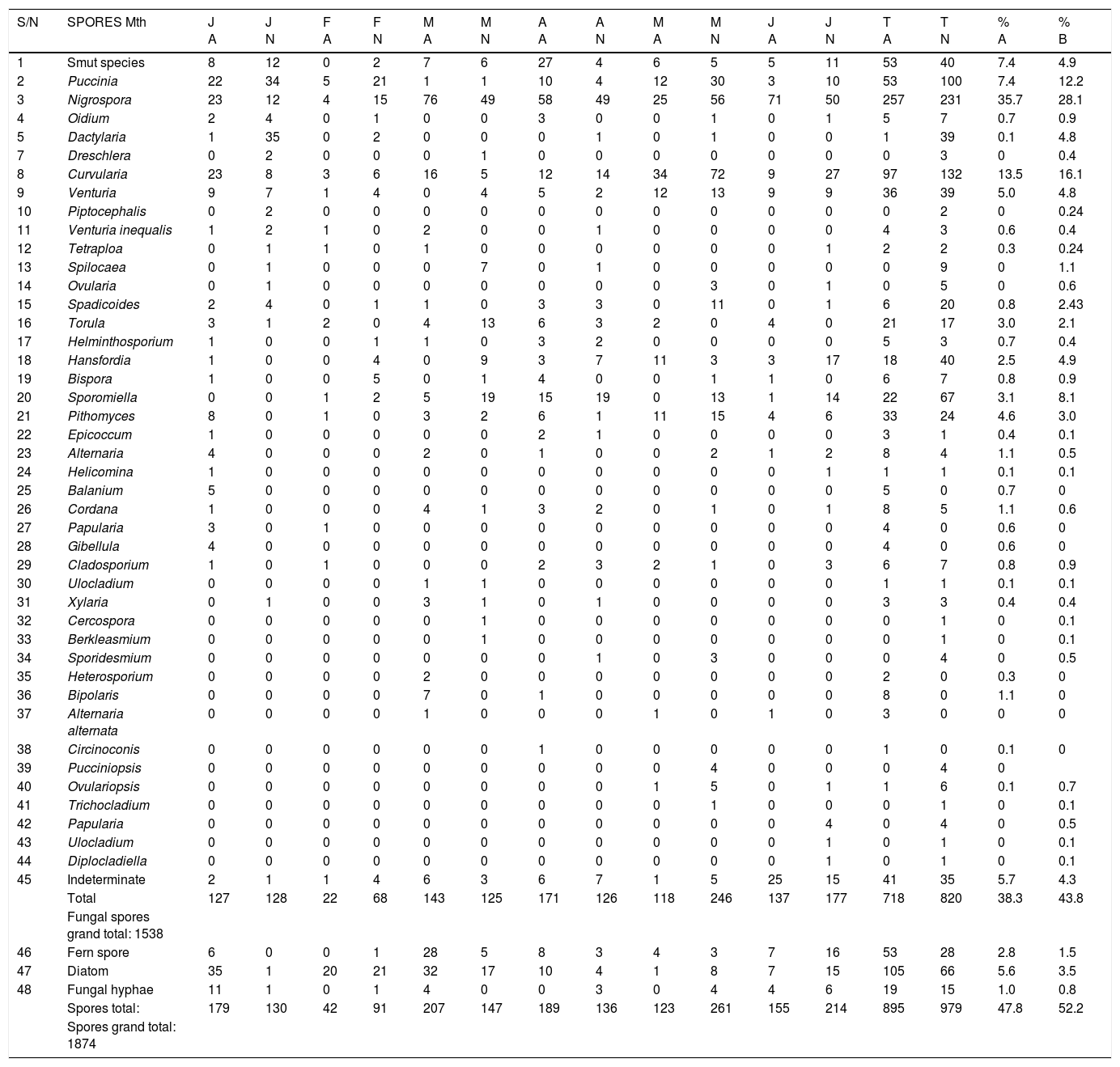
Spores recorded in the study locations were diverse and more quantitatively abundant than pollen counterpart. They were 1874 comprising 47 morphotypes and indeterminate. Abuja had a record of 31 spores type and indeterminate, whereas 38 types and indeterminate were caught in Nasarrawa. Fungal spores 1538 (82.1%) achieved the highest composition among the spores, others were diatom 105 (5.6%) and 66 (3.5%), fern spores 53 (2.8%) and 28(1.5%), fungal hyphae 19 (1.0%) and15 (0.8%) in Abuja and Nassarawa, respectively. Fungal spores of smut species, Puccinia, Nigrospora, Curvularia and Venturia were persistently present in both locations throughout the study period. Spores of Puccinia and Curvularia were more abundant in Nasarawa, whereas Nigrospora spores dominated in Abuja and Nasarawa (Table 2). More aeromycoflora were recorded from Abuja aerosamples in May (246) and June (177), whereas in Nassarawa they dominated in March (143) and April (171). In the dry season (January–March) lower aeromycoflora were encountered (292) and (321) in Abuja and Nassarawa respectively, while more fungal spores morphotype and abundance occurred in the rainy season (April–May) (426) (549) in Abuja and Nassarawa, respectively. Fungal spores which were particularly specific to a location were not encountered. Both locations showed similarity in aeromycoflora morphotypes component with variations in quantity.
Monthly spores count (per 100μL of acetolyzed sample) in study locations.
| S/N | SPORES Mth | J A | J N | F A | F N | M A | M N | A A | A N | M A | M N | J A | J N | T A | T N | % A | % B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Smut species | 8 | 12 | 0 | 2 | 7 | 6 | 27 | 4 | 6 | 5 | 5 | 11 | 53 | 40 | 7.4 | 4.9 |
| 2 | Puccinia | 22 | 34 | 5 | 21 | 1 | 1 | 10 | 4 | 12 | 30 | 3 | 10 | 53 | 100 | 7.4 | 12.2 |
| 3 | Nigrospora | 23 | 12 | 4 | 15 | 76 | 49 | 58 | 49 | 25 | 56 | 71 | 50 | 257 | 231 | 35.7 | 28.1 |
| 4 | Oidium | 2 | 4 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 5 | 7 | 0.7 | 0.9 |
| 5 | Dactylaria | 1 | 35 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 39 | 0.1 | 4.8 |
| 7 | Dreschlera | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0.4 |
| 8 | Curvularia | 23 | 8 | 3 | 6 | 16 | 5 | 12 | 14 | 34 | 72 | 9 | 27 | 97 | 132 | 13.5 | 16.1 |
| 9 | Venturia | 9 | 7 | 1 | 4 | 0 | 4 | 5 | 2 | 12 | 13 | 9 | 9 | 36 | 39 | 5.0 | 4.8 |
| 10 | Piptocephalis | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.24 |
| 11 | Venturia inequalis | 1 | 2 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 | 3 | 0.6 | 0.4 |
| 12 | Tetraploa | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 0.3 | 0.24 |
| 13 | Spilocaea | 0 | 1 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 1.1 |
| 14 | Ovularia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 5 | 0 | 0.6 |
| 15 | Spadicoides | 2 | 4 | 0 | 1 | 1 | 0 | 3 | 3 | 0 | 11 | 0 | 1 | 6 | 20 | 0.8 | 2.43 |
| 16 | Torula | 3 | 1 | 2 | 0 | 4 | 13 | 6 | 3 | 2 | 0 | 4 | 0 | 21 | 17 | 3.0 | 2.1 |
| 17 | Helminthosporium | 1 | 0 | 0 | 1 | 1 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 5 | 3 | 0.7 | 0.4 |
| 18 | Hansfordia | 1 | 0 | 0 | 4 | 0 | 9 | 3 | 7 | 11 | 3 | 3 | 17 | 18 | 40 | 2.5 | 4.9 |
| 19 | Bispora | 1 | 0 | 0 | 5 | 0 | 1 | 4 | 0 | 0 | 1 | 1 | 0 | 6 | 7 | 0.8 | 0.9 |
| 20 | Sporomiella | 0 | 0 | 1 | 2 | 5 | 19 | 15 | 19 | 0 | 13 | 1 | 14 | 22 | 67 | 3.1 | 8.1 |
| 21 | Pithomyces | 8 | 0 | 1 | 0 | 3 | 2 | 6 | 1 | 11 | 15 | 4 | 6 | 33 | 24 | 4.6 | 3.0 |
| 22 | Epicoccum | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 0.4 | 0.1 |
| 23 | Alternaria | 4 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 8 | 4 | 1.1 | 0.5 |
| 24 | Helicomina | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0.1 | 0.1 |
| 25 | Balanium | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0.7 | 0 |
| 26 | Cordana | 1 | 0 | 0 | 0 | 4 | 1 | 3 | 2 | 0 | 1 | 0 | 1 | 8 | 5 | 1.1 | 0.6 |
| 27 | Papularia | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0.6 | 0 |
| 28 | Gibellula | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0.6 | 0 |
| 29 | Cladosporium | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 3 | 2 | 1 | 0 | 3 | 6 | 7 | 0.8 | 0.9 |
| 30 | Ulocladium | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.1 | 0.1 |
| 31 | Xylaria | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 3 | 0.4 | 0.4 |
| 32 | Cercospora | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 33 | Berkleasmium | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 34 | Sporidesmium | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 4 | 0 | 0.5 |
| 35 | Heterosporium | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0.3 | 0 |
| 36 | Bipolaris | 0 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 1.1 | 0 |
| 37 | Alternaria alternata | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| 38 | Circinoconis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 | 0 |
| 39 | Pucciniopsis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | |
| 40 | Ovulariopsis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 1 | 1 | 6 | 0.1 | 0.7 |
| 41 | Trichocladium | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0.1 |
| 42 | Papularia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 0 | 0.5 |
| 43 | Ulocladium | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0.1 |
| 44 | Diplocladiella | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0.1 |
| 45 | Indeterminate | 2 | 1 | 1 | 4 | 6 | 3 | 6 | 7 | 1 | 5 | 25 | 15 | 41 | 35 | 5.7 | 4.3 |
| Total | 127 | 128 | 22 | 68 | 143 | 125 | 171 | 126 | 118 | 246 | 137 | 177 | 718 | 820 | 38.3 | 43.8 | |
| Fungal spores grand total: 1538 | |||||||||||||||||
| 46 | Fern spore | 6 | 0 | 0 | 1 | 28 | 5 | 8 | 3 | 4 | 3 | 7 | 16 | 53 | 28 | 2.8 | 1.5 |
| 47 | Diatom | 35 | 1 | 20 | 21 | 32 | 17 | 10 | 4 | 1 | 8 | 7 | 15 | 105 | 66 | 5.6 | 3.5 |
| 48 | Fungal hyphae | 11 | 1 | 0 | 1 | 4 | 0 | 0 | 3 | 0 | 4 | 4 | 6 | 19 | 15 | 1.0 | 0.8 |
| Spores total: | 179 | 130 | 42 | 91 | 207 | 147 | 189 | 136 | 123 | 261 | 155 | 214 | 895 | 979 | 47.8 | 52.2 | |
| Spores grand total: 1874 |
Mth, Months; J, January; F, February; M, March; A, April; M, May; J, June; L, locations; A, Abuja, N, Nassarawa; T, total.
Diatom 171 (9.1%) were next to fungal spores although low in abundance. They were more dominant in January to April and February to March in Abuja and Nassarawa respectively, which fall within the rainy season. Fern spores were sparsely represented from March to June, within the rainy period, fairly higher than in January and February part of the dry season, Major catch were mainly Nephrolepis type. Fungal hyphae were present, although in low abundance throughout the study period in both locations.
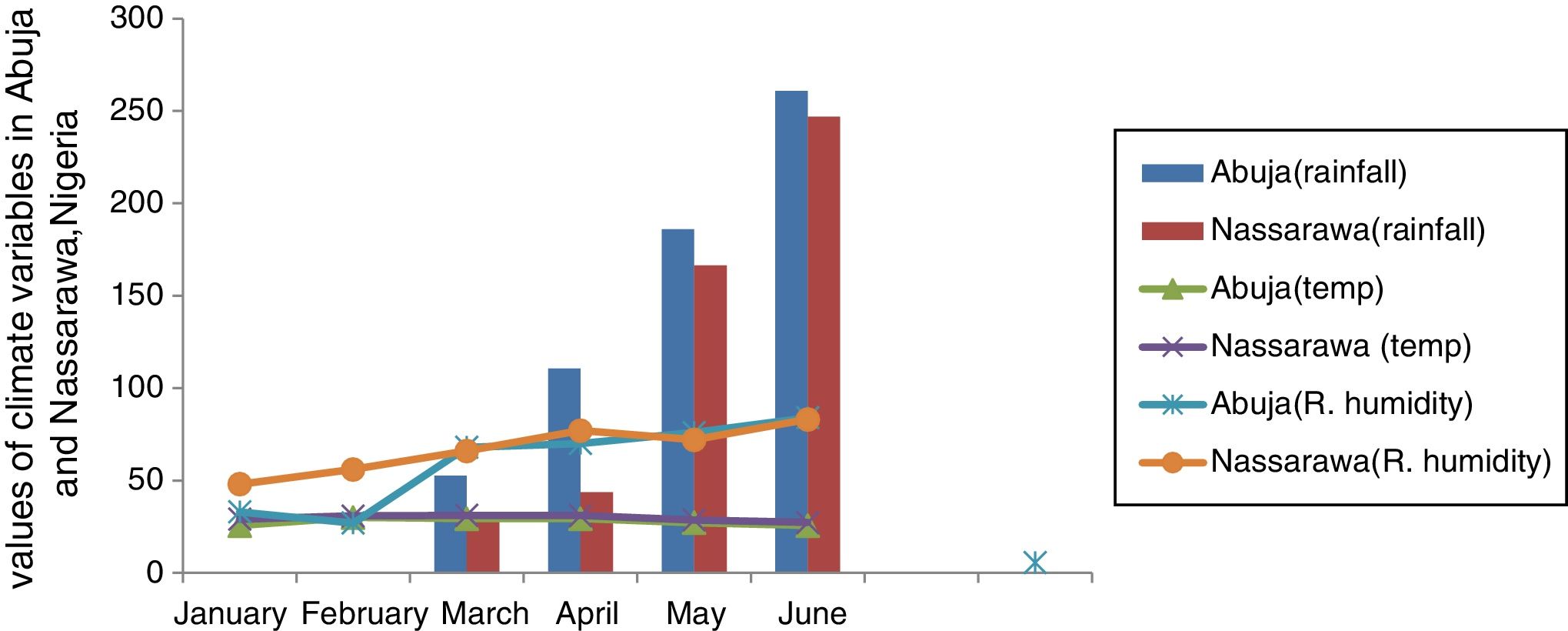
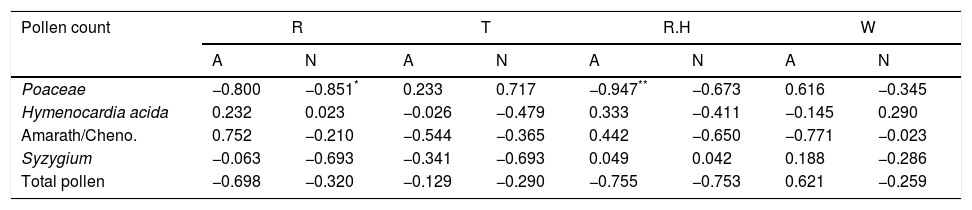
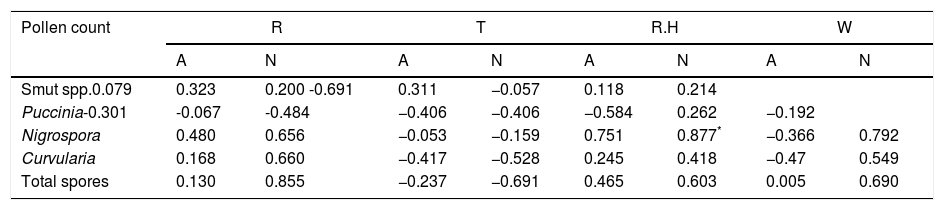
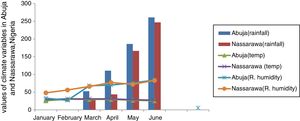
Relationship between weather variables and spatio-temporal pattern of pollen and fungal spores in Abuja and Nassarawa, North-Central NigeriaThe same trend of relationship was found between aeroallergens and weather variables in both study locations. Two different weather extremes were noted: dry period, devoid of rain accentuated dispersal of pollen in the atmosphere, and period of high record of rain which favoured sporulation of fungal spores (Fig. 1). The frequency of the most dominant pollen and total pollen with the exception of Hymenocardia acida and Amarathaceae/Chenopodiaceae correlated positively with wind speed in Abuja, whereas Hymenocardia acida among other dominant pollen correlated positively with wind speed in Nassarawa (Table 3). Total fungal spores frequency and all dominant spores, with the exception of Puccinia correlated positively although not significantly with rainfall in both locations (Table 4, Plate 1).
Correlation coefficients between pollen frequency and climate variables.
| Pollen count | R | T | R.H | W | ||||
|---|---|---|---|---|---|---|---|---|
| A | N | A | N | A | N | A | N | |
| Poaceae | −0.800 | −0.851* | 0.233 | 0.717 | −0.947** | −0.673 | 0.616 | −0.345 |
| Hymenocardia acida | 0.232 | 0.023 | −0.026 | −0.479 | 0.333 | −0.411 | −0.145 | 0.290 |
| Amarath/Cheno. | 0.752 | −0.210 | −0.544 | −0.365 | 0.442 | −0.650 | −0.771 | −0.023 |
| Syzygium | −0.063 | −0.693 | −0.341 | −0.693 | 0.049 | 0.042 | 0.188 | −0.286 |
| Total pollen | −0.698 | −0.320 | −0.129 | −0.290 | −0.755 | −0.753 | 0.621 | −0.259 |
Correlation coefficients between fungal spores frequency and climate variables.
| Pollen count | R | T | R.H | W | ||||
|---|---|---|---|---|---|---|---|---|
| A | N | A | N | A | N | A | N | |
| Smut spp.0.079 | 0.323 | 0.200 -0.691 | 0.311 | −0.057 | 0.118 | 0.214 | ||
| Puccinia-0.301 | -0.067 | -0.484 | −0.406 | −0.406 | −0.584 | 0.262 | −0.192 | |
| Nigrospora | 0.480 | 0.656 | −0.053 | −0.159 | 0.751 | 0.877* | −0.366 | 0.792 |
| Curvularia | 0.168 | 0.660 | −0.417 | −0.528 | 0.245 | 0.418 | −0.47 | 0.549 |
| Total spores | 0.130 | 0.855 | −0.237 | −0.691 | 0.465 | 0.603 | 0.005 | 0.690 |
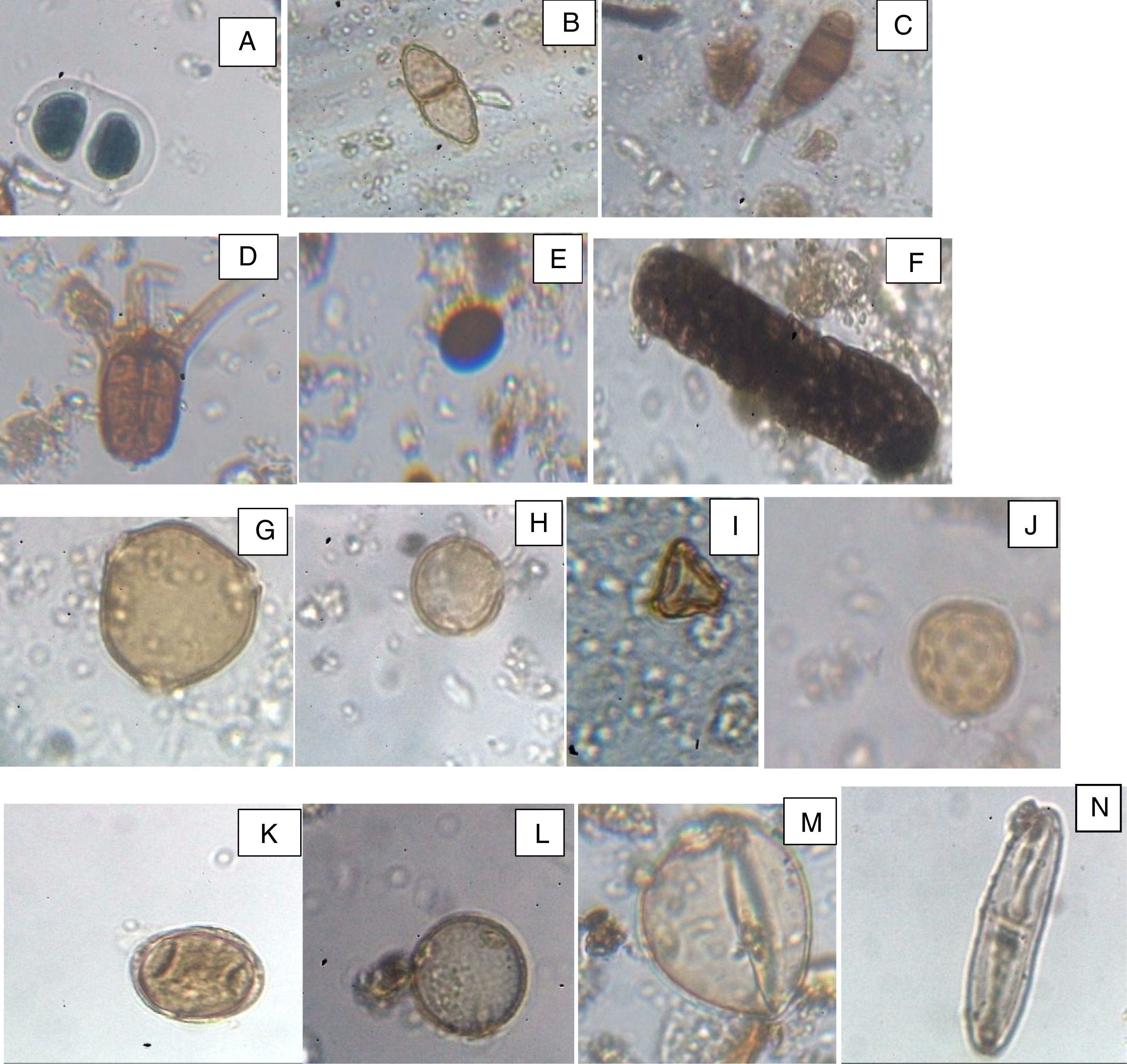
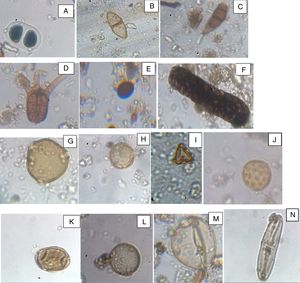
Photomicrography of some aeroallergens: fungal spores: A. Bipolaris, B. Puccinia, C. Curvularia, D. Tetraploa, E. Nigrospora F. Berkleasmium. Pollen grains; G. Pentaclethra macrophylla, H. Lannea welwitschii, I. Syzygium guineense, J. Amarathaceae/Chenopodiaceae, K. Casuarina equisetifolia, L. Hymenocardia acida, M. Poaceae, N. Diatom.
Abuja and Nassarawa States lie contiguous and are both located in North-Central Nigeria. The annual rainfall varies between 1100–1600mm and 1200–1500mm, respectively. The amount of rainfall is much higher than the extreme northwest and northeast but antithetic of rainfall pattern in Coastal and equatorial Nigeria, which have a higher amount and intensity.14,19 Climate variables unequivocally regulated aeroallergen spatio-temporal pattern in ambient atmosphere. In both locations, there were records of diverse pollen and spores morphotype with low quantitative abundance, this could be attributed to the sparseness of vegetation in the study locations and entomophily of major source plants. In comparison to a similar work in Kogi North-Central Nigeria, fewer pollen types with higher quantitative abundance were recorded.14 Differences in composition of aeroallergens trapped could be influenced by the choice of appropriate sampling site. A sampling site free from obstruction, with free flow of air was chosen for the study and could have influenced a diverse input of aeroallergens, although qualitatively into the trap. In a similar study which used the same sampler, high diverse pollen and spores morphotypes and quantity were recorded in Abuja, Nigeria, although with a longer duration (12 calendar months) from 6/1/2011–5/31/2012.11 Pollen concentrations were lower compared to other aeropalynological records in Southeast Nigeria.10,20 Also, lower compared to records in North-Central and Southwest Nigeria.11,21 Some pollen recorded were location-specific, pollen dispersed from Brachystegia eurycoma, Acacia, Ceiba pentandra, Eugenia nodiflora, Gloriosa superba in Nassarawa were related to autochthonous plants source. Slight difference in pollen morphotypes was related to difference in sub-vegetational type.
The occurrence of a higher pollen concentration in the month of December in both locations affirms the role of wind action and dry periods in being more favorable for higher pollen dispersal into the ambient atmosphere. The dry and dusty northeast trade wind (harmattan) that originates from the Sahara Desert and characterized by strong wind usually prevails over northern Nigeria from November to February.22 Airborne pollen were higher in both locations in January and February which falls within the harmattan period. Numerous allergenic diseases such as allergic conjunctivitis, vernal keratoconjunctivitis which are triggered by pollen, have been previously reported to be preponderant in Jos, North-Central Nigeria during the harmattan period.23,24 Harmattan is therefore a season of concern for pollen allergic individuals in the study locations. Knowledge of this association aids anticipation and proper prophylaxis especially for asthmatic patients whose conditions are exacerbated with low level pollen exposure.25 Suspected allergenic pollen at the harmattan period include those of Hymenocardia acida, Poaceae, Amarathaceae/Chenopodiaceae. Several studies have shown relationships between pollen concentration and increased hospital admission for asthma exacerbation and other allergenic diseases.26 Onset of rain reduces pollen concentration in the atmosphere at both locations as rain washes down suspended pollen in the atmosphere.
The rainy period was more favorable for fungi sporulation as aeromycoflora were quantitatively abundant in the rainy months in both locations. Fungal spores were major contributors of the aeroallergen spectrum with those belonging to Deuteromycete dominant. Spores of Puccinia, Smut species, Curvularia and Nigrospora were frequently and abundantly present in both locations. Puccinia and Smut species are pathogenic fungi on cereals and other economic crops. Their frequent preponderance in the atmosphere of the study locations could be a huge threat to economic crops.
Research has shown that most severe and poor control asthma is associated with fungal sensitization.27 Spores of Curvularia and Nigrospora are potent allergen carriers. Both showed a high skin reactivity among atopic patients in Corpus Christi, Texas, USA.28Curvularia is a dematiaceous filamentous fungus, responsible for allergic sinusitis, and bronchopulmonary diseases.29 Nigrospora showed allergenic effect in respiratory allergic patients by intradermal skin sensitivity test in the Terai Area, India.30 The presence of these fungal spores among others could cause debilitating health conditions. Several fungal spores whose concentrations were linked with asthma exacerbations in previous research were present in the atmosphere of the study locations. Such spores include those of Cladosporium, Alternaria alternate, Epicoccum, Helminthosporium, etc.
The present study showed an inextricable regulation of rainfall on aeroallergen concentration especially pollen and spores and provide a baseline information on spatio-temporal relationship of aeroallergens with weather variations and vegetation. The study is important to farmers, allergologists, allergic patients in management of plant diseases and allergic ailments in the study locations. In order to outsmart seasonal allergenic response, reduction of indoor wetness, use of nose masks, sunglasses inter alia during aeroallergen risk periods and possible use of suspected aeroallergens for immunotherapy by allergists is recommended.
Conflict of interestThe authors have no conflict of interest to declare.