INTRODUCTION
Allergic reactions to midriatic eyedrops are rare despite extensively used by ophthalmologists, occurring in only 6 % of total reactions associated to eyedrops1. This may result from preservatives, additives (sodium metabisulfite, benzalkonium chloride, tetracaine, echothiophate iodide) as well as from the drug itself2-6.
Phenylephrine is responsible for 54-96 % of all cases referred in literature7-10.
CASE REPORT
A 56-year-old man, without personal or familial history of allergic diseases, was admitted in 2005 for retinal detachment and was diagnosed left eye melanoma. Two hours after the instillation of phenylephrine 5 %, tropicamide 0.5 % and oxibuprocain eyedrops the patient developed intense conjunctival hyperemia, with erythema and oedema of the eyelids and persistent foreign body sensation. The reaction resolved 8h after treatment unknown to the patient. Because of recurrence of symptoms after instillation of the same drugs, the patient was referred to our outpatient clinic for further investigation. Previously, the patient underwent ocular examination using midriatic eyedrops, uneventfully.
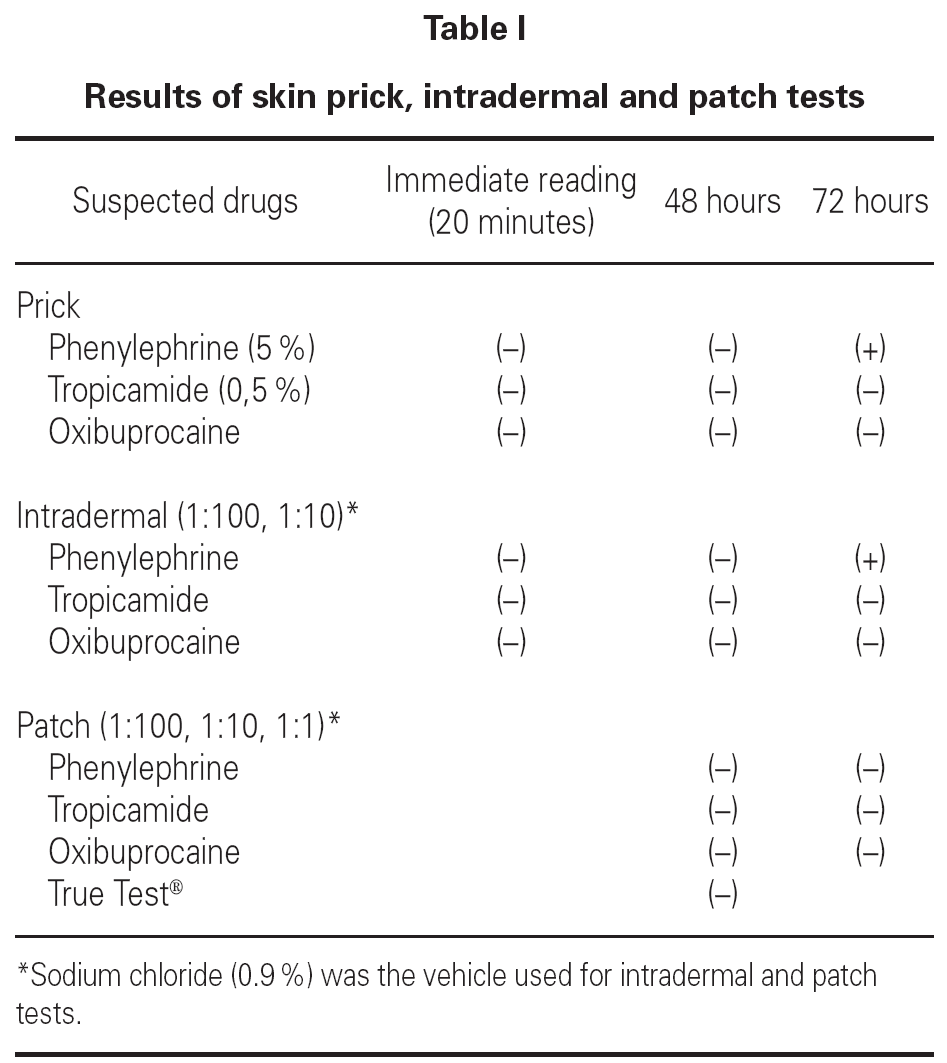
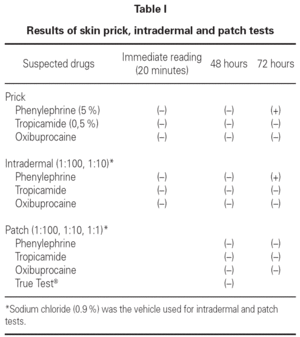
We performed skin prick tests with the commercial formulations of the drugs mentioned above, intradermal tests (1:100 and 1:10 concentrations) and patch tests (1:100, 1:10 and 1:1 concentrations). Patch tests with European standard series (True Test®) were also performed.
At 72 hours skin prick and intradermal tests to phenylephrine were positive (duplication of the mean diameter of the papule). The other results can be seen in table I.
According to the clinical history and results from skin tests we considered diagnostic provocation with phenylephrine unnecessary. The patient had no clinical symptoms with topical instillation of tropicamide and oxibuprocain, latter on.
Phenylephrine seems to be the culprit drug in this case report.
The patient and ophthalmologist were informed to avoid phenylephrine use.
DISCUSSION
Phenylephrine is a sympathomimetic amine that is formed by a benzene ring with an OH radical in position 3 and a lateral chain of etilamine.
It is an extensively used midriatic in ophthalmology that can act as a potent sensitizing agent and can be the cause of allergic contact reactions in exposed patients1-6.
In the study of late cutaneous hypersensitivity reactions associated with phenylephrine, as with other drugs not present in the standardized battery of allergens of the European Society of Contact Dermatitis, there still exists discussion and controversy on the concentrations, vehicles and where patch tests should be performed11,12.
Until this moment, the predictive value of a negative patch test is unknown. In this situation, some authors recommend to perform simultaneous late readings of intradermal tests with culprit drugs.
In this case report all patch tests performed were negative, which can be due to: 1) use of an inadequate vehicle, in this case sodium chloride; 2) low drug concentration and 3) reduced cutaneous absorption.
Although the patient refused to do biopsy of skin lesions, the majority of case reports describe type IV hypersensitivity reactions with perivascular infiltrates of lymphocytes and eosinophils13.
The authors emphasize the relevance of simultaneous late readings of patch and intradermal tests. Therefore, if the latter are positive, they can help in final diagnosis, as in our report.
Correspondence:
Carmen Maria da Costa Botelho
Serviço de Imunoalergologia
Hospital de São João
Alameda Professor Hernâni Monteiro
4200 Porto. Portugal
e-mail: carbotelho@gmail.com