The IL-15/NF-κB axis has an important role in coeliac disease (CD) and may represent a molecular target for immunomodulation. Ascorbate (vitamin C) is known to show inhibitory effects on NF-κB. Therefore, we studied if ascorbate supplementation to gliadin gliadin-stimulated biopsy culture could down-regulate the mucosal immune response to gliadin in CD.
MethodsDuodenal biopsy explants from treated CD patients were gliadin challenged in vitro (100μg/ml) with and without 20mM ascorbate. An extra tissue explant in basal culture was used as internal control. Secretion levels of nitrites (3h), and IFNγ, TNFα, IFNα, IL-17, IL-13, and IL-6 (24h) were measured on the supernatants. IL-15 was assayed by western-blot on whole protein duodenal explants.
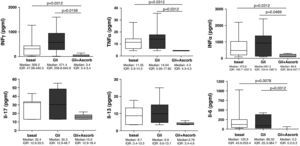
ResultsThe addition of ascorbate to in vitro culture gliadin-challenged biopsies blocked the secretion of nitrites (p=0.013), IFNγ (p=0.0207), TNFα (p=0.0099), IFNα (p=0.0375), and IL-6 (p=0.0036) compared to samples from non-ascorbate supplemented culture. Cytokine secretion was downregulated by ascorbate even to lower values than those observed in basal cultures (IFNγ: p=0.0312; TNFα: p=0.0312; IFNα: p=0.0312; and IL-6: p=0.0078). Gliadin-challenge induced IL-15 production in biopsies from treated CD patients, while the addition of ascorbate to culture medium completely inhibited IL-15 production. Moreover, the inhibition of IL-15 by ascorbate took place even in the only treated CD-patient who had basal IL-15 production.
ConclusionsAscorbate decreases the mucosal inflammatory response to gluten in an intestinal biopsy culture model, so it might have a role in future supplementary therapy in CD.
Coeliac disease (CD) is a common gastro-intestinal disorder caused by a hypersensitivity reaction to wheat gliadin and similar proteins from rye and barley, affecting genetically predisposed individuals (HLA-DQ2/DQ8). The current treatment is a life-long strict gluten-free diet (GFD).1,2
The most accepted model of the CD immunopathogenesis is the two-signal model, which establishes that gliadin has a dual effect on the CD duodenum, triggering the development of an innate immune response in the epithelium, and activating an adaptive immune response controlled by gluten-reactive T cells with a Th1 cytokine profile.3,4 Innate immunity, and specifically interleukin (IL)-15,5,6 plays a key role in the development of CD through a DQ2-independent mechanism.7 The induction of IL-15 seems to be involved in the initial stages of the disease leading to epithelial stress, increase tight-junction permeability, enterocyte apoptosis and dendritic cell (DC) activation,5,6,8–12 facilitating the development of the secondary adaptive response.3 Moreover, the gliadin amplifies the production of inflammatory cytokines through the nuclear factor (NF)-κB13 with a positive feedback by IL-15, which is also a potent NF-κB activator.14 Moreover, DCs are important players in the connection between the innate and the subsequent adaptive immune response,15 and require NF-κB for their development, survival, function and cytokine production.16–18 Thus, the IL-15/NF-κB axis is revealed to have an important role in the pathogenesis of CD and may represent a molecular target for strategies of immunomodulation.19
NF-κB is a heterogeneous collection of dimeric proteins subjected to a complex regulatory mechanism,20,21 involving the inhibitory proteins I-κB that bind to NF-κB subunits which became active after dissociation. This takes place when NF-κB inducers promote I-κB phosphorylation mediated by two I-κB kinases (IKKs), and targets I-κB for its degradation by the 26S proteaseome,22,23 followed by translocation of NF-κB dimmers to the nucleus and elicit their function. It has been recently proposed that ascorbate, may be able to inhibit IKK activation and, therefore, by blocking I-κB phosphorylation, NF-κB cannot translocate and bind to its DNA targets.24 These inhibitory properties of ascorbate can be elicited at concentrations of 20mM, intracellularly in vivo, without showing toxic effects to cells, or inhibiting other inducible factors.
Nitric oxide (NO) is involved in the histological changes produced in coeliac disease. In the mouse monocyte/macrophage cell line RAW 264.7, pre-challenged with IFNγ, the gliadin is able to enhance the NF-κB activity and iNOS protein expression and therefore NO production. Both effects were reduced by NF-κB activation inhibitors, thereby indicating that gliadin should modulate iNOS gene expression through NFKB activation.25
Given the role of the NF-κB pathway in the pathogenesis of CD, we wondered whether ascorbate has an effect on the inhibition of the early/innate immune response triggered by gluten and, therefore, can be used as a supplementary therapeutical strategy to GFD on CD patients. To address this question we have cultured biopsies from treated CD patients stimulated in vitro with gliadin, with and without supplementation of ascorbate. Our data confirm that ascorbate inhibits the gliadin-induced expression of IL-15 in CD biopsy explants.
Materials and methodsStudy subjectsWe studied eight CD patients treated on a GFD for a minimum of six months (mean age 41.7yrs, range 23–68yrs, 25.0% males) and three non-CD healthy controls (mean age 63.6yrs, range 61–68yrs, 0.0% males). The diagnosis of CD was based on compatible symptoms, positive serology (IgA antiendomysial or antitransglutaminase antibodies), positive genetic markers (HLA-DQ2/8), and mucosal changes in the duodenum. At the time of sample collection, CD patients had mucosal recovery (Marsh 0-1) and negative serology for at least 1yr. Healthy controls were referred to the gastroenterology clinics due to other intestinal diseases which were later ruled out, and no mucosal alterations were found in the duodenum. All patients were attended in the adult gastroenterology clinics from the “Hospital Clínico Universitario”, Valladolid, Spain, as part of routine diagnostic procedures. Informed consent was obtained from patients, and the study protocol was approved by the Ethics Committee from both “Hospital Clínico Universitario” and Faculty of Medicine, University of Valladolid.
Biopsy cultureThree intestinal biopsy explants were collected from each individual and cultured in vitro as previously described.26 Briefly, all biopsies were collected in ice-chilled PBS containing 0.1% gentamicine and cultured within 1h in RPMI 1640 supplemented with 10% heat-inactivated FBS, penicillin (100U/ml), streptomycin (100μg/ml) and fungizone (0.25μg/ml) (all from Cambrex Iberia Products, Barcelona, Spain). One sample from each individual antiendomysial cultured in basal medium was used as an internal control. One explant from each patient was challenged in vitro with a gliadin solution (100μg/ml) (Sigma, St Louis, MO, USA) for only 3h, which is considered normal exposure and concentration in the duodenum after a meal. A second explant was co-cultured both with gliadin (100μg/ml) and 20mM of ascorbate (Sigma), a potent non-toxic NF-κB inhibitor.24 After 3h, biopsy explants specimens were washed up in PBS containing 0.1% gentamicine and later cultured for another 21h in new clean culture medium to determine whether gliadin challenge is followed by a secondary response. Tissue culture was carried out in vitro by immersion in culture dishes placed in a cell incubator with 5% CO2 at 37°C. After 24h (3h with stimulus+21h with basal medium), tissue was embedded in RNAlater (Ambion, Applied Bisystems, Austin, TX, USA) and snap-frozen until protein extraction using the TRIZOL® Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Supernatants were collected at both 3 and 24h. All reagents were checked and discarded for lypopolisaccharide (LPS) contamination with Lymulus amebocyte lisate, PYROGENT® Plus (Cambrex) (Detection limit 0.06EU/ml).
Effector molecules on culture supernatantsBiopsy culture supernatants after 3h of culture were assayed for the concentration of oxidative stress by applying the Griess reaction following manufacturer's instructions (Molecular Probes, Invitrogen) (detection limit (D.L.) 1μM). The nitric oxide is very unstable, therefore the Griess reaction measures the total amount of nitrites, which are primary metabolites derived from the instantaneous oxidation of NO. Supernatants at 24h of culture were also analysed using a multiplex assay on a Luminex TM platform (BioRad, Hercules, CA, USA), for the concentration of IFNγ [D.L. 3.38pg/ml], tumour necrosis factor α (TNFα) [D.L=4.331pg/ml], IFNα [D.L=89.87pg/ml], IL-17 [D.L=12.98pg/ml], IL-13 [D.L=3.49pg/ml] and IL-6 [D.L=0.19pg/ml].
Western-blot analysisFrom whole biopsy explants, 8μg of protein isolated was added per well. They were separated by using a 15% acrilamide/bisacrilamide (37.5:1) gel in a mini-Protean II (BioRad), and later transferred onto PVDF membranes of 0.45 Micron (Pierce Biotechnology Inc. IL, USA). Membranes were incubated with primary specific antibodies to human IL-15 (mouse monoclonal MAB247) (R&D, Minneapolis, MN, USA) at a final dilution of 1/400, performing a second incubation with antibodies to mouse IgG labelled with horseradish peroxidase (Amersham Biosciences Europe, Freiburg, Germany). Chemiluminiscent substrate Lumigen PS-3 (Amersham) and autoradiography film Hyperfilm ECL (Amersham) were used for developing. Recombinant human IL-15 (Peprotech, London, UK) was used as a positive control.
Statistical analysisThe Friedman test was applied in all cases to compare different culture conditions from the same patient (non-parametric and paired two-tailed test). The secondary Wilcoxon matched paired test between pairs of conditions was only applied in those cases where the Friedman test was statistically significant. The level of significance was fixed at p<0.05.
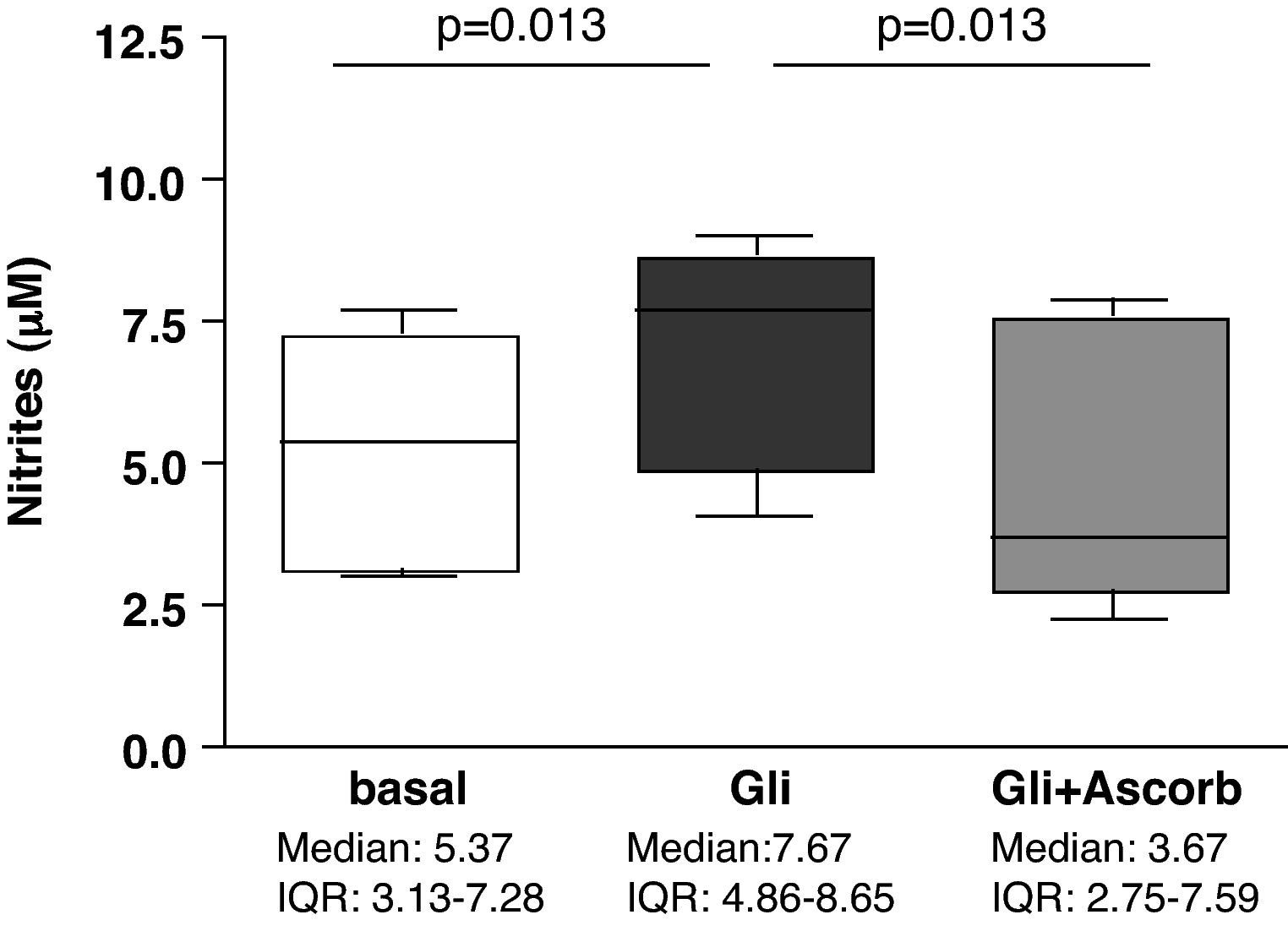
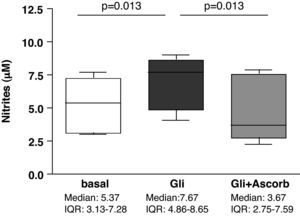
ResultsAscorbate blocks the secretion of nitrites induced by gliadin challenge in biopsy samples from CD patientsStatistically significant differences were found in nitrites secretion after 3h of culture when explants from the same patient were compared in basal conditions and after gliadin-challenge, both with and without ascorbate supplementation (p<0.001). Gliadin challenge increased the secretion of nitrites, therefore indicating an increase in the production of NO in duodenal explants from treated CD patients (p<0.05) (Fig. 1) compared to non-challenged explants from the same patient as previously described.27,28 The addition of ascorbate, at a non-toxic concentration of 20mM, to gliadin-challenged cultures effectively blocked the induction of nitrite secretion by gliadin (p<0.05) (Fig. 1). Because ascorbate is also a strong antioxidant, and given that the Griess reaction is based on the determination of nitrites (mainly derived from the oxidation of NO), these results could reflect an experimental artefact. To confirm that ascorbate really blocks the immune response to gliadin, we have also studied the secretion of cytokines on culture supernatants.
Biopsy culture secretion of nitrites in eight treated CD patients after 3h of gliadin challenge (100μg/ml) with (Gli+Ascorb) and without (Gli) ascorbate supplementation (20mM), compared to basal culture (all at 3h). Statistically significant differences are shown (p<0.05, Wilcoxon matched paired test). Horizontal bars indicate median and whiskers maximum and minimum values. IQR: interquartile range.
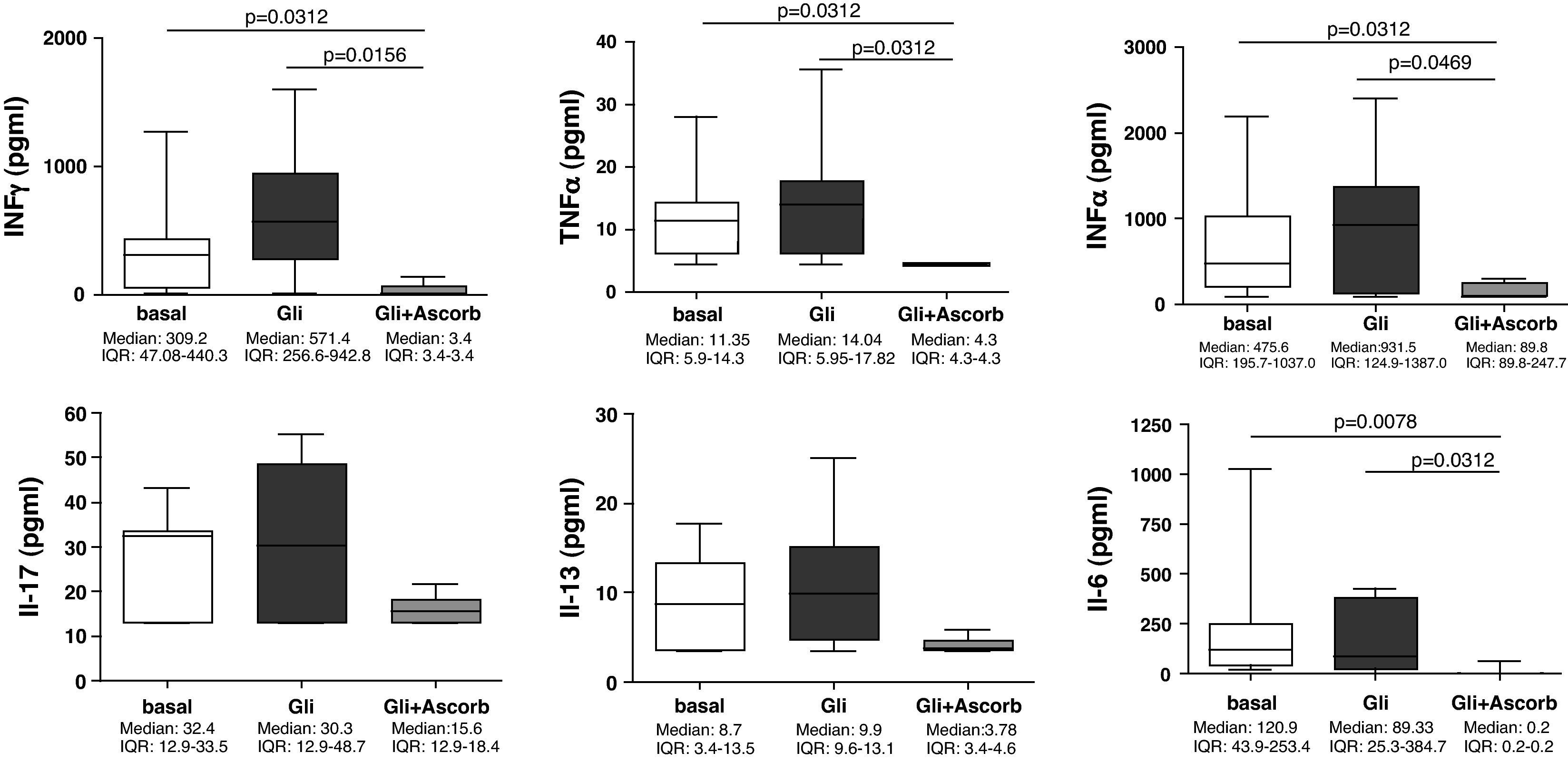
Our findings showed that in the supernatants at 24h (3h of challenge with gliadin plus 21h of basal culture) none of the assayed cytokines were statistically increased compared to the basal culture. However, the addition of ascorbate to culture medium, for those 3h of challenge, clearly decreased the secretion of all cytokines, compared to non-ascorbate supplemented gliadin-challenged supernatants (Fig. 2). The decrease was statistically significant for IFNγ (p=0.0156), TNFα (p=0.0312), IFNα (p=0.0469) and IL-6 (p=0.0312). Moreover, in these cases cytokine secretion was downregulated even to lower values than those observed in basal cultures (IFNγ: p=0.0312; TNFα: p=0.0312; IFNα: p=0.0312; and IL-6: p=0.0078) (Fig. 2), thereby confirming its inhibitory properties.
Supernatants biopsy culture secretion of IFNγ, TNF-α, IFNα, IL-17, IL-13, and IL-6 in eight treated CD patients after gliadin challenge (100μg/ml) with (Gli+Ascorb) and without (Gli) ascorbate supplementation (20mM) (3h of challenge and 21h of basal culture) compared to the basal culture. Statistically significant differences are shown (p<0.05, Wilcoxon matched paired test). Horizontal bars indicate median and whiskers maximum and minimum values. IQR: interquartile range.
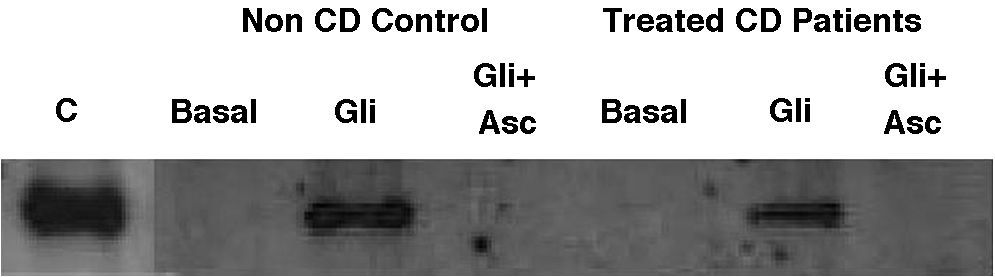
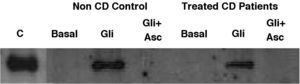
Basal IL-15 production in biopsy explants was only detectable in one out of eight treated-CD patients and was absent in all three non-CD controls (Fig. 3). After gliadin-challenge, as previously described,6,29,30 gliadin induced IL-15 production in treated-CD patients. In this assay it was detected in seven out of eight cases (Fig. 3). Similar results were obtained in non-CD controls, where gliadin was also revealed as an IL-15 inducer in all three non-CD cases compared to basal culture, as previously reported by our group.30 Gliadin-induced IL-15 had detectable levels even 21h after gliadin had been removed from culture medium, given that western-blot was performed at the end of the culture (3h of gliadin challenge and 21h in basal condition). As expected, the addition of ascorbate to culture medium completely inhibited IL-15 production not only by CD biopsy explants but also by those from non-CD controls. Moreover, it is noteworthy that the IL-15 inhibition by ascorbate took place even in the only treated CD-patient who had basal IL-15 production.
Representative western-blot analysis using whole protein biopsy explants of non-CD controls and treated-CD patients, after 24h of basal culture (Basal), and after 3h of gliadin challenge (100μg/ml) with (Gli+Asc) and without (Gli) ascorbate supplementation (20mM) and 21h of basal culture. C: human recombinant IL-15 lane.
Basal IL-15 was only detected in one CD patient. Gliadin induced IL-15 production in both non-CD controls (three out of three) and treated CD patients (seven out of eight). Ascorbate inhibited IL-15 production in all cases, even in a patient who had detectable basal levels of IL-15.
In this study we have shown, by using a culture model of duodenal explants, that the addition of ascorbate to culture medium decreases the secretion of inflammatory mediators in response to gluten in CD patients. Supplementation of ascorbate, of a non-toxic concentration of 20mM, to gliadin challenged biopsy culture not only inhibits the gliadin-induced production of nitrites, but also downregulates the secretion of proinflammatory cytokines (IFNγ, TNFα, IFNα and IL-6), and completely inhibits that of IL15.
Although it has been largely reported that gliadin is a potent cytokine inducer in CD patients by using culture models – especially in the cases of IFNγ and TNFα,4,27 no statistically significant upregulation of cytokine expression was observed in any of the culture supernatants (Fig. 2). The explanation to this discrepancy probably resides in the experimental design, where the time of challenge in culture (3h, which is considered a normal exposure and concentration in the duodenum after a meal, followed by 21 extra hours in basal conditions) was lower than previous studies in which challenge was performed for 24h. In this situation, it is reasonable to think that the total level of cytokine released should be smaller than previous studies.
Gliadin is capable of increasing the production of NO and this is related to the expression of iNOS. Additionally, gliadin increased the binding activity of NF-κB/DNA, the degradation of IκBα and the nuclear translocation of p50 and p65 subunits.25
With these findings we have confirmed that by using a non-toxic (20mM) supplementation of ascorbate to biopsy culture challenged with gliadin, the gliadin-induced production of nitrites is inhibited. The effects of gliadin are probably mediated by iNOS, and ascorbate acts blocking its pathway.25,31 The expression levels of the cytokines IFNγ, TNFα, IFNα, and IL-6 are decreased to below those levels observed in basal cultures (Fig. 2). This decrease could be the result of the inhibition of the NF-κB pathway induced by ascorbate.
Moreover, ascorbate also affects the IL-15 pathway. This property of ascorbate is very interesting since IL15 is considered to be a central cytokine in CD immunopathogenesis given its capacity to initiate the innate immune response to gliadin6,29 and to activate dendritic cells,15,16,32 therefore facilitating the development of the secondary adaptive response.3
Ascorbate is capable of inhibiting DC activation, blocking cytokine secretion and the immunostimulatory properties. Moreover, ascorbate-treated DCs are able to generate regulatory T cells with FoxP3+ expression.33 Future studies should address whether ascorbate is also capable of inhibiting DCs maturation in the duodenum of CD patients and even its capacity to generate gliadin specific regulatory T cells.33
It has been recently shown that oral supplementation of ascorbate attenuates several anaphylactic reactions to soybean glycinin-induced hypersensitivity on a swine model.34 In humans, oral supplementation has been reported to delay the progression of transplant-associated coronary arteriosclerosis,35 and of cardiac transplant-associated arteriosclerosis,36 and even reduces xenobiotic-induced T-cell hyperactivation.37 Ascorbate is also capable of inhibit phytohaemagglutinin and concanavalin A mitogen-stimulated peripheral blood mononuclear cells by suppressing both formation and release of IFNγ.38 All together, these results clearly point to the possible therapeutical use of ascorbate in diseases, such as CD. However, further studies are needed to confirm this, including double-blind placebo-controlled trails aiming to characterise not only the optimal dose in vivo of ascorbate, but also the safe amount of gluten intake tolerated by patients on an ascorbate trial.
As a final remark, this property of ascorbate of modulating the immunological response to gluten could be used as a supplement to the GFD. Considering the residual amounts of gluten that some “free gluten products” still have, ascorbate could be a necessary supplement to the dietary treatment of CD. Given the relevance of our findings, this effect should be further studied in order to confirm these results, and specifically to analyse how ascorbate exerts its immunomodulatory effects on DCs, given that they are central players in the immune response to gluten in CD.
Conflict of interestThe authors have no conflict of interest.
Spanish Ministry of Education (FPU, AP2002-2696), Junta de Castilla y León (FPI fellowship), Universidad de Valladolid (FPI fellowship), Instituto de Salud Carlos III, Spanish Ministry of Health (PI 070244 and CES08/016), Fundación IECSCYL, Junta de Castilla y León (SAN-GRS232/B/08), and Phadia Spain. We also thank Ms. Alicia Ortega for her technical help.