Pulmonary disease is a frequent acute and chronic manifestation in sickle cell disease (SCD), presenting high morbidity and mortality.
ObjectivesTo identify the prevalence and association of asthma, allergic sensitization and altered pulmonary function in patients with SCD (SS and Sβo).
MethodsA single-center, cross-sectional study was conducted, in which 70 patients with SCD and 44 controls, aged six to 18 years, responded to the questionnaire of the International Study of Asthma and Allergies in Childhood (ISAAC), complemented with an anamnesis regarding the associated clinical outcomes. All patients underwent immediate hypersensitivity skin tests with aeroallergens and a pulmonary function evaluation (spirometry). Regarding the statistical analysis, parametric and non-parametric methods were used, depending on the variables studied. Tests were considered significant when p<0.05.
ResultsThere was no significant difference between the patients and controls regarding the prevalence of asthma and allergic sensitization (p>0.05). The number of occurrences of acute chest syndrome per patient per year was significantly higher for asthmatic patients than for non-asthmatic patients (p=0.04). Obstructive pulmonary function occurred in 30.9% of the patients and in 5.4% of the controls, and restrictive pulmonary function occurred in 5.5% of the patients and 5.4% of the controls. Asthma and wheezing in the last 12months had significant associations with obstructive pulmonary function (p=0.014 and p=0.027, respectively).
ConclusionsThe occurrence of asthma, allergic sensitization and alteration in lung function in patients with SCD reinforces the importance of routine monitoring of these diagnoses, which allows for early treatment and prevention of the evolution of pulmonary disease in adulthood.
Sickle cell disease (SCD) is a chronic hemolytic anemia with acute and chronic manifestations and high morbidity/mortality, and pulmonary disease is a frequent manifestation.1,2 Acute chest syndrome (ACS) is the most common form of acute pulmonary disease in SCD and is defined as a new segmental opacity on a chest radiograph, other than atelectasis, accompanied by respiratory symptoms and/or fever.2 Asthma is a lung disease characterized by bronchial hyperreactivity and reversible bronchoconstriction, with chronic inflammation and airway remodeling.3
In SCD, the association of asthma with ACS is common, and the mechanisms involved are complex and multifactorial.4 The exacerbation of asthma and ACS are commonly associated, and there is difficulty in separating one situation from the other clinically. The features of these two clinical situations are confusing, since they may present similar symptoms, such as wheezing and obstructive pulmonary function changes. In addition, the timeframes of the two conditions may overlap, opening the possibility for worsening of bronchial hyperreactivity and asthma during the critical period of ACS.5
The diagnosis of asthma is based on classic clinical symptoms and findings and should be complemented by pulmonary function tests and allergy skin testing.6 In epidemiological studies, the prevalence of asthma can be assessed through a validated questionnaire, including that of the International Study of Asthma and Allergies in Childhood (ISAAC).7 Review studies on pulmonary function assessments in adult patients with SCD have shown a predominance of the restrictive pattern, whereas in children there is a greater prevalence of obstructive pattern abnormalities.8
Currently, only a very limited number of studies have evaluated the prevalence of asthma in patients with SCD. An adequate diagnosis of SCD patients and asthma symptoms includes a clinical history, pulmonary function tests (PF) and allergy skin tests for allergic sensitization screening. A limited number of studies have performed such evaluations and included clinical data, lung function and allergic sensitization. In these studies, the prevalence of allergic sensitization ranged from 27 to 45%.9–11
Based on these observations, the objective of this study was to identify the prevalence of asthma, allergic sensitization and alteration in lung function in children and adolescents with sickle cell disease and to compare these findings with a control group of children and adolescents without sickle cell disease.
Patients and methodsThis was a single-center cross-sectional study conducted at the Federal University of São Paulo (Brazil) that included sickle cell disease (SCD) patients (HbSS and HbSβo) who had their diagnosis confirmed by hemoglobin electrophoresis - isoelectric focusing and/or high-performance liquid chromatography and controls (children and adolescents without SCD, who underwent neonatal screening for hemoglobinopathies-AF, and hemoglobin electrophoresis). Therefore, an SCD patient group (n=70) was statistically compared with a group of controls (n=44), aged six to 18 years old, with the same socio-economic status. All subjects answered the asthma module of the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire, supplemented with an anamnesis directed at the associated clinical outcomes. All patients and controls underwent skin prick tests with aeroallergens and an evaluation of pulmonary function using spirometry. Laboratory tests, such as a hemogram and lactate dehydrogenase (LDH) analysis, and clinical data regarding the frequency of ACS, hospitalizations and the use of hydroxyurea were collected.
Spirometry measures a number of parameters, including forced vital capacity (FVC), and the volumes and flows from it. In most cases, patients with obstructive pulmonary disease have a reduced FEV1, being normal or reduced in restrictive diseases with reduced FVC. This parameter is useful for assessing airway permeability and is very sensitive for obstructive disorders, even in asymptomatic patients.12
Exclusion criteriaAt the time of data collection, patients should be at baseline with no history of infections and painful crises for at least two months and no blood transfusion for at least four weeks. Spirometry assessments were not performed in patients who failed to undergo the test, even after two non-consecutive trials. In these situations, the patients were excluded from the study.
ISAAC questionnaireThe SCD patients were evaluated for the possibility of an association with asthma. The standard questionnaire of the ISAAC study was applied with the eight questionnaire questions regarding asthma, complemented with an anamnesis directed at the clinical outcomes associated with asthma,13 such as the onset of symptoms, the frequency of hospitalizations and visits to the emergency room due to respiratory disease, and the use of oral corticosteroids and specific medications for asthma in the last year. Both the questionnaire and the anamnesis were carried out by the principal investigator.
Allergic sensitizationAllergic sensitization research was performed by using skin prick tests to investigate allergic sensitization (according to existing recommendations) by two allergy physicians.14 The tests were performed in the absence of antihistamines for at least two weeks. In addition to the positive (histamine) and negative (saline) controls, the following allergens were tested: Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, cat epithelium, dog epithelium, fungi mix, Blatella germanica, and Periplaneta americana. Those with an average papule diameter greater than 3mm were considered sensitized in the absence of a response to the negative control.
Pulmonary function evaluationA pulmonary function evaluation was performed by spirometry, according to the American Thoracic Society (ATS),15 by two trained physiotherapists, with certified equipment (Masterscreen™, Carefusion), after calibration (3L syringe, ± 2% variation). The following parameters were analyzed: FVC, FEV1, the relationship between FVC and FEV1, and FEF 25−75%. The patient was then given bronchodilator medication (inhaled salbutamol, 400 mcg), and new curves were obtained after 15min of rest. Bronchodilator was suspended prior to the test and the patient was instructed to discontinue any asthma-specific treatment on the day of the exam.
Sample sizeThe sample size was calculated according to the study by Sylvester et al.,16 assuming values (mean±SD) for FEV1 of 1.5±0.5L in the sickle cell disease group and of 1.9±0.7L in the control group. For a sample power of 90% and a significance level of 5%, with a ratio of two patients to one control, it would be necessary to include at least 37 controls and 75 patients.
Statistical analysisQuantitative characteristics were described according to groups using summary measures (mean, standard deviation) and compared between groups using Student’s t-tests. Qualitative characteristics were described according to groups using absolute and relative frequencies, and their association was verified with chi-squared tests, Fisher’s exact tests or likelihood ratio tests.17
Among the patients with SCD, treatment and genotype associations were verified according to asthma, wheezing, allergic sensitization and pulmonary function using chi-squares tests, Fisher’s exact tests or likelihood ratio tests. Quantitative traits were described according to the occurrence or absence of asthma and compared with the use of Student’s t-tests, and pulmonary function was compared with the use of analysis of variance (ANOVA).17
The data were tabulated using Microsoft Excel 2003 software, and the analysis was performed using IBM-SPSS for Windows version 20.0 software. The tests were performed with a significance level of 5% (p≤0.05). The study was approved by the Research Ethics Committee of the Federal University of São Paulo/Hospital São Paulo/ Brazil (CAAE: 20633413.4.0000.5505), and informed, written parental consent was obtained.
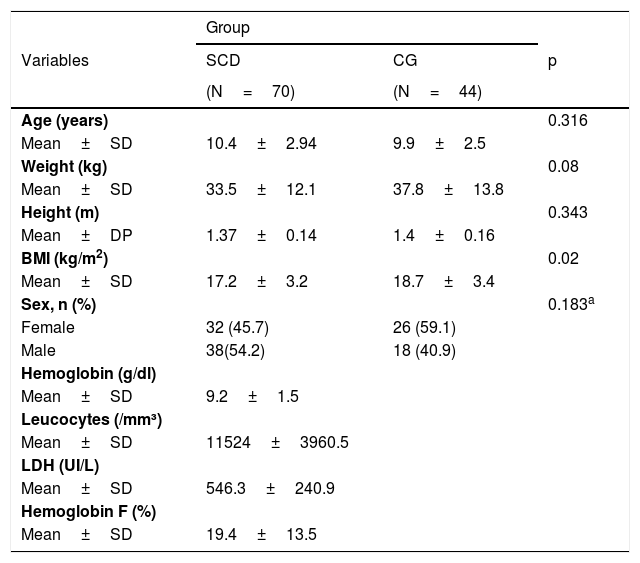
ResultsSeventy children and adolescents with SCD (63 HbSS and seven HbSβo) and 44 controls performed the procedures adequately and were included in the study. Regarding demographics, patients with SCD were similar to those in the control group (CG). Table 1 shows the demographic and laboratory characteristics of patients with SCD and the CG.
Demographics and lab results for the sickle cell disease (SCD) group and control group (CG).
| Group | |||
|---|---|---|---|
| Variables | SCD | CG | p |
| (N=70) | (N=44) | ||
| Age (years) | 0.316 | ||
| Mean±SD | 10.4±2.94 | 9.9±2.5 | |
| Weight (kg) | 0.08 | ||
| Mean±SD | 33.5±12.1 | 37.8±13.8 | |
| Height (m) | 0.343 | ||
| Mean±DP | 1.37±0.14 | 1.4±0.16 | |
| BMI (kg/m2) | 0.02 | ||
| Mean±SD | 17.2±3.2 | 18.7±3.4 | |
| Sex, n (%) | 0.183a | ||
| Female | 32 (45.7) | 26 (59.1) | |
| Male | 38(54.2) | 18 (40.9) | |
| Hemoglobin (g/dl) | |||
| Mean±SD | 9.2±1.5 | ||
| Leucocytes (/mm³) | |||
| Mean±SD | 11524±3960.5 | ||
| LDH (UI/L) | |||
| Mean±SD | 546.3±240.9 | ||
| Hemoglobin F (%) | |||
| Mean±SD | 19.4±13.5 | ||
t-Student’s t test; test; LDH, Lactate dehydrogenase; SD, Standard deviation.
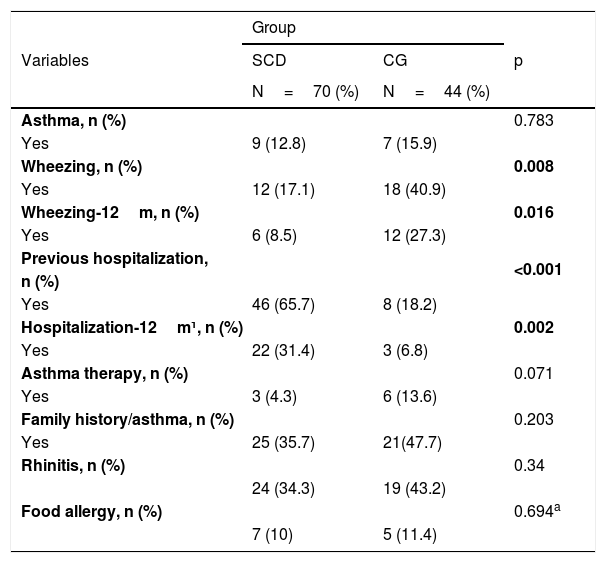
Table 2 demonstrates the respiratory symptoms, the diagnosis of asthma and their respective morbidities. The prevalence of asthma diagnosis in SCD was 12.8%, and in the CG, it was 15.9% (p=0.783). A total of 4.3% of patients with SCD and 13.6% of CG were in asthma treatment with inhaled corticosteroids. The lifetime frequency of wheezing, dry cough at night, and use of steroids in the last 12months were lower in the SCD group (p<0.05) than in the control group. The frequencies of previous hospitalizations and hospitalizations in the last 12months due to respiratory disease were statistically higher in the SCD group (p<0.001 and p=0.002, respectively) than in the control group.
Asthma and respiratory outcomes (ISAAC questionnaire complemented with anamnesis) for sickle cell disease (SCD) patients and the control group (CG).
| Group | |||
|---|---|---|---|
| Variables | SCD | CG | p |
| N=70 (%) | N=44 (%) | ||
| Asthma, n (%) | 0.783 | ||
| Yes | 9 (12.8) | 7 (15.9) | |
| Wheezing, n (%) | 0.008 | ||
| Yes | 12 (17.1) | 18 (40.9) | |
| Wheezing-12m, n (%) | 0.016 | ||
| Yes | 6 (8.5) | 12 (27.3) | |
| Previous hospitalization, | <0.001 | ||
| n (%) | |||
| Yes | 46 (65.7) | 8 (18.2) | |
| Hospitalization-12m¹, n (%) | 0.002 | ||
| Yes | 22 (31.4) | 3 (6.8) | |
| Asthma therapy, n (%) | 0.071 | ||
| Yes | 3 (4.3) | 6 (13.6) | |
| Family history/asthma, n (%) | 0.203 | ||
| Yes | 25 (35.7) | 21(47.7) | |
| Rhinitis, n (%) | 0.34 | ||
| 24 (34.3) | 19 (43.2) | ||
| Food allergy, n (%) | 0.694a | ||
| 7 (10) | 5 (11.4) | ||
Chi-squared test. 1- respiratory event.
Regarding ACS, the total number of events per patient ranged from zero to 12, with an average of 2.83±2.91 events. In the last 12months, ACS occurred 0.39±0.64 times on average, ranging from zero to three events. When considering the number of occurrences of ACS per patient per year (number of events/age of the patient in years), the mean was 0.27±0.27, with a minimum of zero and a maximum of 1.33. The number of occurrences of ACS per patient per year (number of events/age of the patient in years) was significantly higher in asthmatic patients (mean: 0.44±0.44) than in non-asthmatic patients (mean: 0.25±0.23; p=0.04; data not shown).
There was no statistical significance between the laboratory parameters and the presence or absence of asthma (data not shown).
Skin prick tests were positive for one or more allergens in 29 patients (41.4%) with SCD and in 14 individuals (31.8%) from the CG (p=0.328). Twenty-one patients (30%) in the SCD group tested positive for at least two allergens as did nine individuals (20.5%) in the CG (0.284). Additionally, skin prick tests were frequently positive for mites in both groups. The diagnosis of asthma and/or the presence of wheezing in the last 12months was not associated with positive skin prick tests, either for one allergen or for two or more allergens, in either group (p>0.05) (data not shown).
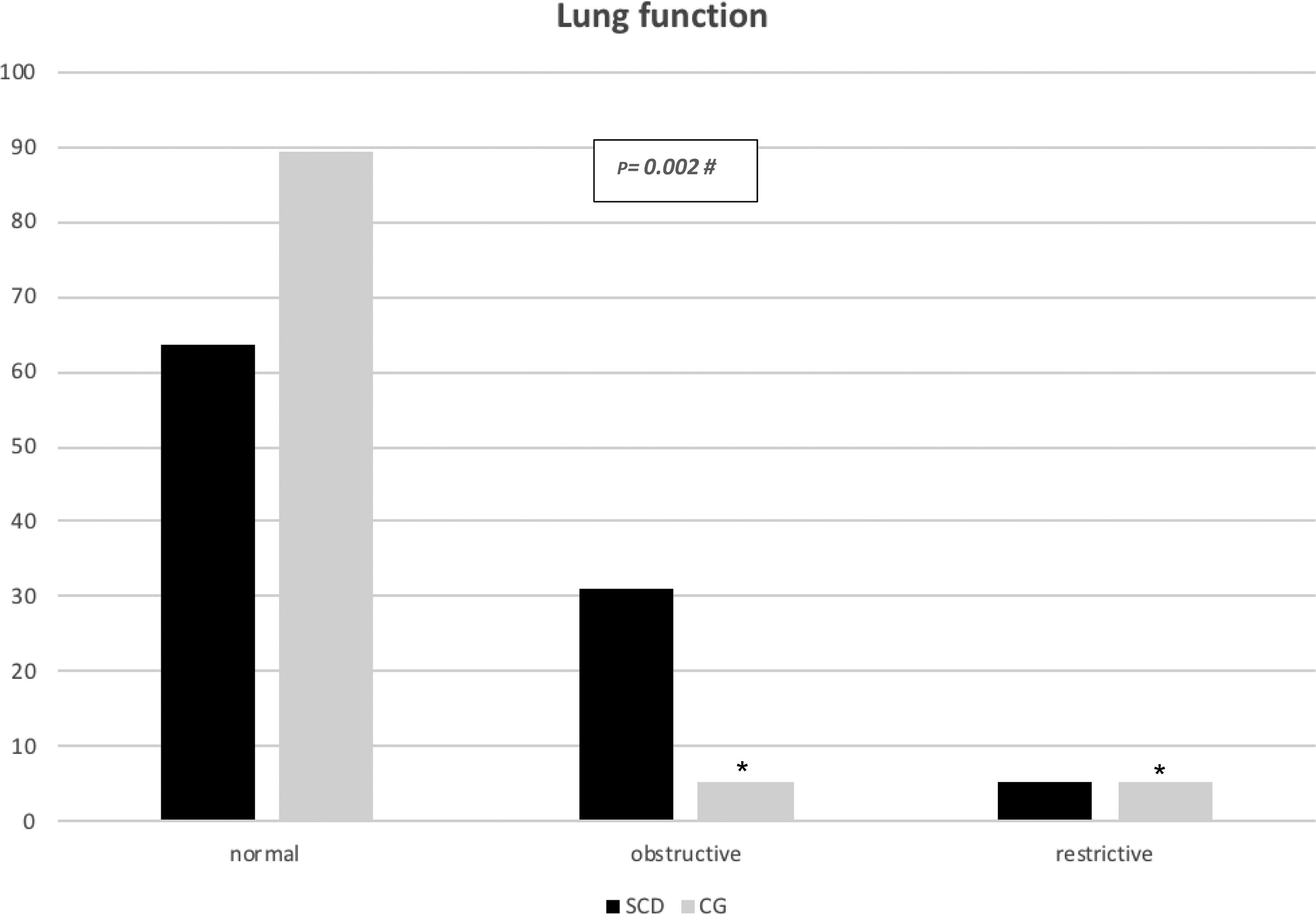
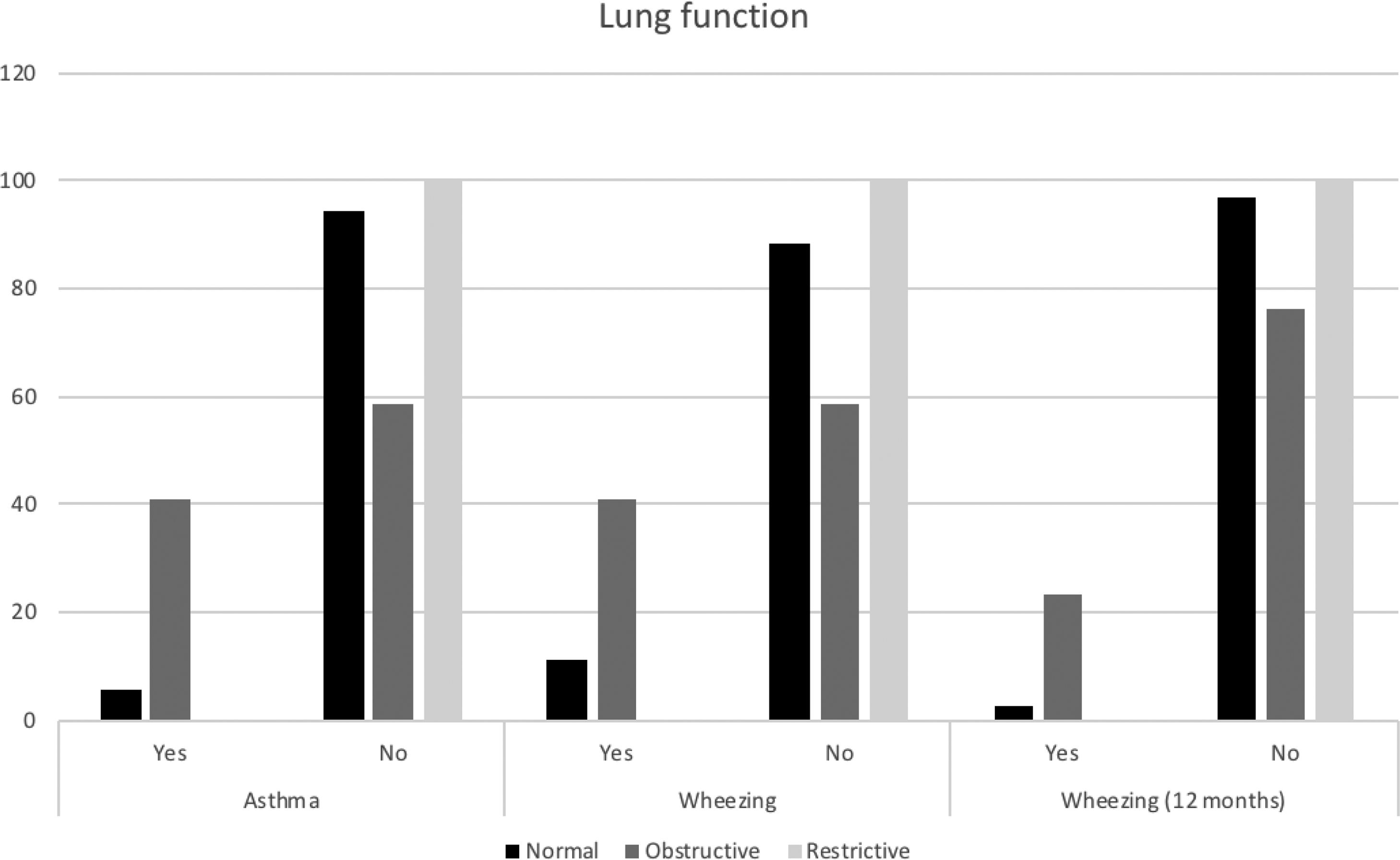
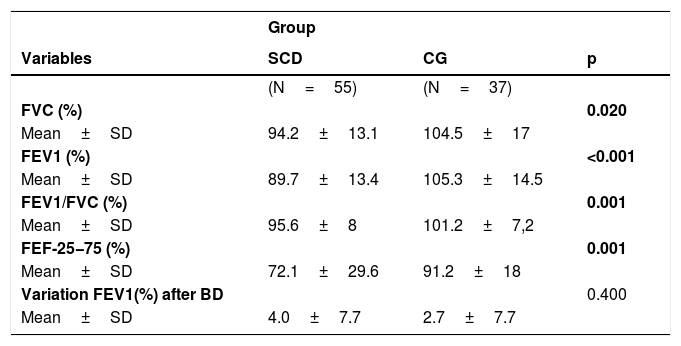
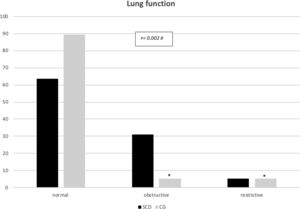
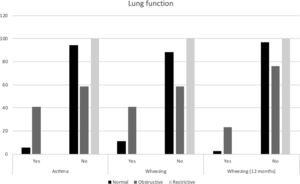
All major spirometry parameters of the SCD group were significantly lower than those of the CG, and the magnitude of response to the bronchodilator was not significantly different between the two groups (Table 3). The frequency of bronchodilator response was similar between the two groups: 9.0% (5/55) in the SCD group and 8.1% (3/37) in the CG. Abnormal lung function was found in 36.4% of the SCD group and 10.8% of the CG group. Obstructive alterations were evidenced in 30.9% of those with SCD and 5.4% of those in the CG, and restrictive alterations were present in 5.5% of those with SCD and 5.4% of those in the CG. Pulmonary function was significantly worse in patients with SCD (p=0.002), as shown in Fig. 1. There was no association between body mass index and spirometry values in patients with SCD and in the CG (data not shown). When analyzing the association of pulmonary function and laboratory parameters, such as ACS and the occurrence of asthma, wheezing and allergic sensitization in the SCD group, we observed that only asthma, wheezing and wheezing in the last 12months were more frequently observed in patients with an obstructive pulmonary pattern than in patients with a restrictive pulmonary pattern (Fig. 2).
Values of the spirometry parameters for the sickle cell disease (SCD) patients and control group (CG).
| Group | |||
|---|---|---|---|
| Variables | SCD | CG | p |
| (N=55) | (N=37) | ||
| FVC (%) | 0.020 | ||
| Mean±SD | 94.2±13.1 | 104.5±17 | |
| FEV1 (%) | <0.001 | ||
| Mean±SD | 89.7±13.4 | 105.3±14.5 | |
| FEV1/FVC (%) | 0.001 | ||
| Mean±SD | 95.6±8 | 101.2±7,2 | |
| FEF-25−75 (%) | 0.001 | ||
| Mean±SD | 72.1±29.6 | 91.2±18 | |
| Variation FEV1(%) after BD | 0.400 | ||
| Mean±SD | 4.0±7.7 | 2.7±7.7 |
Student's t test; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; FEF25−75%: forced expiratory flow between 25 and 75% of forced vital capacity; BD: bronchodilator; SD: standard deviation.
Regarding specific treatment for SCD, 42 (60%) patients had been using hydroxyurea for more than 12months. Twelve (17%) were in chronic transfusion for more than 12months, and 16 patients (22.9%) had neither treatment. There was no significant difference among patients in the different treatments (hydroxyurea, chronic transfusion or neither treatment) regarding the presence or absence of asthma, wheezing, wheezing in the last 12months, allergic sensitization and pulmonary function (data not shown).
DiscussionThe pathophysiology of asthma, ACS and chronic lung disease in SCD correlate in several aspects. Acute asthma exacerbations are confounded with ACS events, and patients with SCD can present with cough, bronchial hyperreactivity and wheezing, even in the absence of an asthma diagnosis.5
Asthma has a significant prevalence in SCD, and in two multicenter studies, this prevalence occurred in 16–28% of participants with SCD.11,18 In the study by Boyd (2006),18 there is no reference in relation to the questionnaire used, and in Strunk (2014),11 the three main factors associated with asthma were family history, wheezing and wheezing after physical exercise. It is worth mentioning that the questionnaire used was the ATS Division of Lung Disease,15 where the symptoms are evaluated in the presence of respiratory infections.
In our study, the prevalence of the diagnosis of asthma was 12.8% in the SCD group, similar to the CG, which did not differ from the studies of SCD that used the ISAAC questionnaire,19,20 and was similar to that observed in children and adolescents in Brazil.7
In the study by Knight-Madden (2005),9 a higher prevalence of asthma was found in patients with SCD than in the control group (48% vs. 22%). It should be noted that although that study used the ISAAC questionnaire, it made a change in the specific question for the diagnosis of asthma, where instead of directly asking if the patient had the diagnosis of asthma, patients were asked if they could have a "touch of asthma".
The choice of diverse tools for assessing the prevalence of asthma, with non-similar questions, can cause variability in the prevalence outcomes. The medical underdiagnosis of asthma is frequent, and when several criteria for the definition of asthma in questionnaires are compared, the result of the direct question about the diagnosis of asthma is usually less prevalent than that of wheezing in the last year. We should also consider the difficulty patients and their families, as well as the physicians who attend this population, have in recognizing asthma. The results of this study confirm the presence of asthma in 12.8% of the patients, but only 4.3% were on asthma therapy.
The presence of wheezing, regardless of the diagnosis of asthma, has been important in the identification of patients with asthma and bronchial hyperreactivity. This is because many patients mention the occurrence of "wheezing", unrelated to the diagnosis of asthma. It should be emphasized that bronchial hyperreactivity may occur more or less intensely, in the same person and at different times and is only one of the mechanisms that contributes to the clinical expression of airflow obstruction.7 In the present study, a prevalence of wheezing of 17.1% was found in the SCD group, regardless of the presence or absence of colds. The study by Cook (2013)19 also evaluated patients aged seven to 16 years with an HbSS (sickle cell anemia) diagnosis using the ISAAC questionnaire, and the presence of wheezing, regardless of the diagnosis of asthma, was 16.7%. A similar result was observed in a study with 163 patients with SCD and a control group with 96 children. The frequency of wheezing associated with a cold, regardless of the diagnosis of asthma, was 17.5% in the SCD group of 2.1% in the control group.21
Early identification of asthma in SCD is critical since the diagnostic criteria are confused and aggravated in the presence of ACS. Boyd (2006)18, in the Cooperative Study of Sickle Cell Disease (CSSCD), examined 291 children with SCD (SS) and showed that asthma was associated with more frequent ACS episodes and that asthmatic children were twice as likely to have ACS when compared to non-asthmatic children (0.39 versus 0.20 episodes per patient per year). Glassberg (2012)22 also found 0.08 ACS episodes per patient per year in asthmatic patients versus 0.04 ACS episodes per patient per year in non-asthmatic patients. The present study has similar results, with 0.44±0.44 ACS episodes per patient per year in asthmatic patients and 0.25±0.23 ACS episodes per patient per year in non-asthmatic patients. The number of ACS episodes was higher in asthmatic patients than in non-asthmatic patients (p=0.04). This finding corroborates the importance of early diagnosis and the finding that patients with SCD had a significantly higher number of previous hospitalizations and hospitalizations in the last 12months due to respiratory diseases than CG. These findings confirm the importance of investigating the relationship between ACS and asthma and identifying and diagnosing patients for the purpose of providing adequate asthma control and decreasing ACS events. In our study, only 4.3% of patients with SCD were undergoing treatment for asthma before the investigation began. Pahl (2016)23 found that ACS was more common in SCD genotypes SS and Sβo and that ACS was more frequent in asthmatic patients. Most patients with ACS or asthma failed to receive formal consultation services from pulmonary subspecialists.
The association between bronchial hyperreactivity and higher levels of LDH in children with SCD may suggest an interaction between pulmonary blood vessel hemolysis and airway responsiveness.24 However, Glassberg (2012)22 found no difference between LDH values in wheezing and non-wheezing SCD patients. In our study, although patients with SCD had increased values of LDH, in the analysis of the laboratory parameters of patients with and without asthma, there was also no significant difference in any of the laboratory parameters.
Since the association of asthma and atopy is already well established in patients without a diagnosis of SCD, this investigation of this association is necessary in patients with SCD, with the possibility that it will assist in the diagnosis of asthma.7,25
In the study by DeBaun (2014),10 allergic sensitization to two or more allergens occurred in 27.7% of patients with SCD and was associated with a greater risk of future ACS events. One asthma symptom was also associated with future ACS events. This prevalence is similar to the results of the present study, with a prevalence of skin prick test positivity for two or more allergens in 30.0% of SCD patients; however, in our study, there was no association between allergic sensitization and a greater number of ACS. In any case, we suggest the evaluation of allergic sensitivity in patients with suspected asthma.
Four articles have been published relating allergic sensitization to aeroallergens and asthma in SCD, two of which found a higher prevalence of positive skin prick tests among asthmatics, as in the present study, where the presence of positive skin prick tests for two or more allergens prevailed among asthmatics.9,11 The third study, by Field (2011),26 examined bronchial hyperreactivity in patients with SCD and found no association with atopy. The study by Willen (2018)27 demonstrated that aeroallergen sensitization was associated with future ACS. This confirms the need to identify allergic sensitization in patients with SCD and a history of wheezing and/or a diagnosis of asthma, since the presence of atopy is an important risk factor for asthma and allergic sensitization assists in this diagnosis.
The diagnosis of asthma and/or the presence of wheezing or the presence of wheezing in the last 12months showed no association with positive skin prick tests in any of the groups (p>0.05), even when comparing the SCD group and the CG; however, the diagnosis of atopic asthma was higher in this group of patients with SCD (13.7%) than in the healthy individuals from the South of Brazil, who were also analyzed by the ISAAC study.7 It is worth noting that a limitation of this study is that our patient population is part of a convenience sample from a single treatment center and does not represent all children with SCD.
Pulmonary disease in SCD can be compromised with ACS and/or with the presence of asthma, subsequently causing dyspnea, decreased physical capacity, loss of lung function and pulmonary hypertension.28 In children with SCD and altered lung function, although the results are controversial, most studies point to a predominance of the obstructive pattern, and as patients progress to adulthood, the restrictive pattern begins to prevail, demonstrating the evolution to pulmonary chronic disease.29–33
In the present study, it was noted that abnormal pulmonary function was more frequent in the SCD group (36.4%) than in the CG group (10.8%) (p=0.002), and the obstructive pattern was the most prevalent in the SCD group (30.9%), confirming the data of other authors.29–33 In the CG, the obstructive pattern occurred in 5.4% of the children, probably due to the occurrence of asthma in this group of children and adolescents. The presence of asthma, wheezing, and wheezing in the last 12months were common among children with SCD and concomitant obstructive abnormalities in pulmonary function (41.1%, 41.1%, and 23.5%, respectively). The study by Arleta (2014)31 observed that in sickle cell patients with an obstructive pattern of impairment in spirometry, 44% had a history of asthma or wheezing. In a cohort of 1016 children from the Silent Cerebral Infarct Transfusion (SIT) trial, participants with asthma had an increased incidence of ACS episodes compared with those without asthma (19 vs. 12 episodes per 100 patients year).34 The Sleep and Asthma Cohort (SAC) enrolled 252 children with SCD and followed them prospectively for a median of 4.6 years, and they found that a diagnosis of asthma was associated with an increased incidence of ACS episodes.11 Willen et al. (2019) analyzed the CSSCD,18 SIT34 and SAC11 cohorts, resulting in a dataset with 1685 participants, with 390 asthmatic patients (23,1%), and found a positive association with ACS episodes.32 These findings confirm the influence of asthma on the pulmonary disease of patients with SCD, in addition to the influence of recurrent ACS and pulmonary inflammatory disease.
In relation to the treatment in the SCD group, the prescription of hydroxyurea and a chronic transfusion program are therapeutic interventions that positively affect the morbidity and mortality of patients with SCD, as well as in the diagnosis and evolution of asthma. In the present study, there was no significant difference between the different treatments for SCD and the presence or absence of asthma, wheezing, allergic sensitization and alteration in lung function. We must remember that we are considering children and adolescents, and this therapeutic intervention can influence long-term prognosis, with beneficial effects in adulthood.
Agrawal (2019) performed a study with clinical case scenarios on the diagnosis and management of SCD pulmonary manifestations in children and showed that there is variability in the diagnosis and management of SCD airway inflammation among pediatric pulmonologists.35
Numerous studies suggest the relationship between lung disease and a worse prognosis in SCD patients, raising the question of the need for follow-up with a subspecialist (pulmonary or allergy) for asthma control therapy and to reduce the occurrence of ACS, in those patients with two or more episodes of ACS, a family history asthma, a history of atopy (eczema or aeroallergy sensitization), wheezing and exercise intolerance or chronic cough.32 The American Thoracic Society propose to focus on training the future generation of pediatric and adult pulmonologists to develop a group of experts who can work together to improve the evolution of lung disease in SCD patients.36
The limitations of this study are the inclusion of children exclusively with HbSS and Hb Sβo, thus making the results not generalizable to children with other SCD genotypes, not performing plethysmography and being a cross-sectional study. Due to the small sample size, it was not possible to carry out the multivariate analysis. Pulmonary circulation is probably an important factor in the respiratory symptoms of SCD patients, mainly due to ischemic episodes, and the echocardiographic determination of right ventricular and pulmonary artery pressures would be important.
The strengths of this study are that the comparisons are between a studied group and a control group of the same origin, that the patients were evaluated in the same laboratory under similar conditions and that in the literature, to our knowledge, there are only four published articles relating allergic sensitization to aeroallergens and asthma in SCD.
In view of all the aspects discussed, it can be considered that asthma and bronchial hyperreactivity play an important role in SCD patients. Considering that pulmonary disease is one of the most important causes of morbidity in children and mortality in adults with SCD, this study confirms the need to investigate pulmonary pathologies in patients with SCD to provide early therapeutic interventions.
FundingFundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) project: 2013/09185-3.
Conflict of interestThe authors have no conflict of interest to declare.