INTRODUCTION
Crustaceans are a well-known source of food allergens. Hypersensitivy reactions described as a consequence of this seafood consumption include oropharyngeal pruritus, urticaria, angioedema, gastrointestinal distress and anaphylaxis. Barnacle (Pollicipes cornucopiae) is a type of sedentary marine crustacean with a free-swimming larvae. In adult phase it has six pairs of thrunk limbs and the shell is developed into a mantle. In Japan barnacles are used as fertilizer (1). A species of 30-centimetres-barnacle from the cirripeda genus is regarded as delicacy in South America and stalked barnacle is commonly eaten in parts of France and Northwestern Spain. Until now, no adverse reactions have been described in patients consuming this seafood. We report a group of five patients who after ingestion of barnacles presented different kinds of allergic reactions.
MATERIAL AND METHODS
Patients
Case 1. A 6-years-old girl suffered from angioedema in her face, throat itching, disphagia, disphony, dyspnea and wheezing immediately after eating barnacle. She was quickly transferred to an emergency unit where she was treated with adrenaline and metilprednisolone and was kept under observation. The symptoms disappeared within 1-2 hours without further therapy. The patient had personal history of a seasonal rhinoconjunctivitis.
Case 2. A 30-years-old patient developed generalized urticaria and dyspnea, 30 minutes after consumption of lobster, barnacles and turbot. Some months later, he experienced the same symptoms after eating exclusively barnacles. He is sensitized to house-dust-mite and pollens.
Case 3. A 26-years-old patient who noticed oropharyngeal and lip itching, swelling of his lips and urticaria around the mouth several minutes after eating barnacle. He had also noticed oropharyngeal itching when he inhaled the barnacle boiled steam. He suffered from house-dust-mite allergy and was treated with specificic immunotherapy.
Case 4. A 16-years-old boy who experienced lip angioedema and oral itching, several minutes after ingesting boiled barnacles. The symptoms disappeared some hours later without therapy. He had asthma due to house-dust-mite.
Case 5. A 12-years-old girl who had conjunctivitis and angioedema in her face several minutes after ingestion of a barnacle. The symptoms disappeared some hours later without therapy. She has not personal atopic history.
In vivo tests
Skin test. Prick to prick test was performed with boiled and raw barnacle; briefly, a standardized lancet was pricked into boiled and raw barnacle and immediately thereafter into the skin. Skin-prick-tests (SPTs) were performed acording to Pepys' method using histamine (10 mg/ml) and saline solution 0.9 % (in 50 % glycerol), as positive and negative controls respectively. If reaction occurred after 15 minutes, the transverse and vertical diameter of the wheal were measured and mean values recorded. Wheals of 3 mm or greater were regarded as positive in the absence of reaction to negative control. Prick to prick tests to barnacle were performed in ten atopic and non-atopic control population obtaining negative results.
In vitro tests
Barnacle extracts. Barnacles were cut into pieces and homogenized in Phosphate buffer 10 mM, NaCl 150 mM, pH 7 and their proteins were extracted by magnetic stirring in the same buffer (2 h at room temperature). The sample was centrifugated at 5600 g for 30 minutes and the supernatant was dialyzed against water and later filtered and freeze dried.
EAST (Enzyme AllergoSorbent Test). Specific IgE determination. Specific-IgE levels were calculated using BIAL-ARISTEGUI discs. Briefly the liofilized allergen extracts were coupled (10 mg/mL) to the 6-mm diameter CNBr-activated paper discs and EAST was performed with HY-TEC-EIA kit for specific-IgE, following the manufacturer's instructions (Hycor-Biomedical Inc.).
SDS-PAGE. This technique was performed according to the method of Laemmli (2) in a discontinuous gel (running: 12.5 %; stacking: 4 %). The samples were dissolved in 0.125 M HCl-Tris, pH 6,8 and were dissociated with 0.1 % SDS and 5 % beta-mercaptoethanol at 100 °C for 5 minutes. After electrophoresis, gels were stained by diffusion in 0.1 % Coomassie Brilliant Blue R-250 dissolved in methanol/acetic/distilled water (4:1:5). Destaining was also performed by diffusion in the same mixture without dye.
SDS-PAGE Immunoblotting. The proteins were electrophoretically transferred to a polyvinylidene membrane (Immobilo-P membranes) essentially as described by Towin et al. (3). The membrane was rinsed in PBS (Phosphate buffer 25 mM, NaCl 100 mM pH 7) and blocked with 0.05 % Tween 20 in PBS (1 h at 37 °C). After three washes with PBS containing 0.02 % Tween 20, the blots were incubated overnight with 0.5 ml of the patients' sera. Later on, the blots were washed 3 times with PBS and incubated with peroxidase-conjugated rabbit immunoglobulins to human ε-chains. Bound peroxidase was detected by chemiluminescence method according to the manufacturer' instructions (Western Blotting ECL + Plus. Amersham Life Science).
Oral challenges were considered initially for all patients. In one case, medical reasons advised against the test. Other patient was reluctant to undergo the test as he had developed general urticaria and dyspnea every time he had eaten barnacle, and finally the test was cancelled. Two other patients refused and the only one who accepted initially did not undergo it.
RESULTS
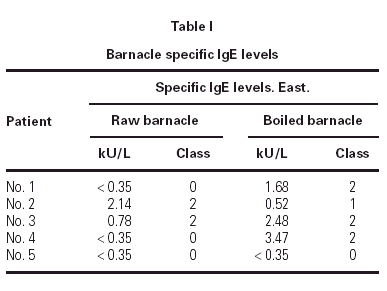
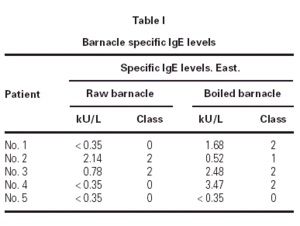
The prick to prick test to barnacle produced wheals from four to ten millimetres of diameter. The barnacle specific-IgE values are summarized in table I.
The prick to prick and specific IgE values to barnacle were correlated in all our patients but one. Patient number five had RAST class 0 for raw and boiled barnacle and however SPT for raw and boiled barnacle was positive.
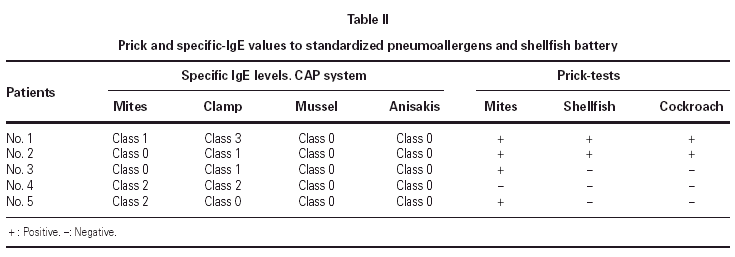
The prick-tests performed with standardized pneumoallergens and shellfish battery, showed that four patients had positive prick-tests against house-dust-mite, two of them had positive prick-tests against shellfish and cockroach and only one patient had positive prick-tests against the three previous allergens. The CAP-system (Pharmacia) was class 2 to house-dust-mites in two patients and class 1 in other one. This system was employed in the specific-IgE antibodies determination of clamp, mussel and Anisakis simplex. A patient had CAP-system class 2 for clamp, two of them had CAP-system class 1 and another one had CAP-system class 3. In all the patients, mussel and Anisakis simplex specific-IgE antibodies were negative. The results are summarized in table II.
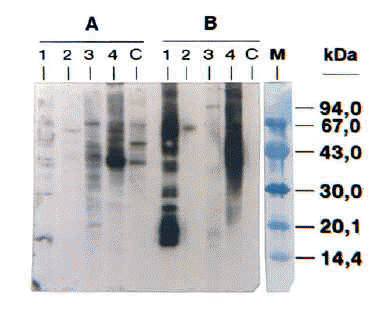
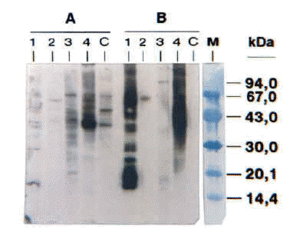
The SDS-PAGE Immunoblotting results showed IgE-binding bands whose molecular masses ranged between 58-68 kDa and 37-39 kDa (fig. 1). This figure shows the IgE-binding bands of cases 1, 2, 3 and 5. Unfortunately, it was not possible to make a photo of case number 4 blotting.
Figure 1.--SDS-PAGE Immunoblotting. A, Raw barnacle extract B, Boiled barnacle extract. Lanes 1, 2, 3, and 4: Patients' sera (cases 1, 2, 3 and 5 respectively). C, Negative control (pool from non atopic subjects' sera). M, Molecular mass markers.
DISCUSSION
Barnacle is considered a delicacy in Spain, however there are no reported cases about sensitization by barnacle in the international literature, apart from those reported by the authors in national meetings (4, 5). In the present study we describe five cases of patients, with different ages, who suffered from urticaria and angioedema after eating barnacle.
Prick to prick positive results indicate that all our patients were sensitized to both raw and boiled barnacle. However we only found measurable specific-IgE levels to boiled barnacle extract in four patients sera. The IgE-binding components from the barnacle extract were studied by SDS-PAGE immunoblotting, obtaining a relevant IgE-binding zone within 58-68 kDa. Although an allergen of similar molecular mass (70 kDa) was described by Leung et al in oyster (Crassostea gigas) (6), this is the first time an allergen from barnacle is described. The western blotting results also showed an IgE binding band of 37-39 kDa, in boiled and raw barnacle extracts. This is the same molecular mass of tropomyosin, a well-known and widespread allergen in arthropods (7, 8) which is the cause of many cases of cross-reactivity between house-dust-mites and other arthropods (crustaceans, insects) sensitization. Significantly most of our patients were sensitized to house-dust-mite. One of them suffered from asthma so he had been treated with immunotherapy with good results. All the patients have eaten all types of crustaceans and molluscs without adverse reaction, after the allergic reaction by barnacle.
In summary, it can be concluded that barnacle could induce IgE-mediated adverse reaction. Our study demonstrates the presence of an IgE-binding protein of 58-68 kDa in barnacle extract. This could be a specific allergen from barnacle as no IgE-binding band with the same molecular mass has been revealed in western blotting of other mollusc and crustacean extracts incubated with the patients' sera. However, further experiments using a larger variety of mollusc and crustacean extracts should be carried out to confirm this proteic band is really a specific allergen from barnacle.