House dust mites are important indoor allergens and are commonly related with asthma and allergic diseases, especially in children. Recently, domestic mite fauna in high altitude (2,500–2,800m) cities of Ecuador have been studied and demonstrated a frequent and abundant presence of mites and their allergens in house dust samples collected.1 Also, other studies had suggested that D. pteronyssinus and D. farinae are important sensitising agents in patients with allergic rhinoconjunctivitis and bronchial asthma in Quito.2 Their significance is greater than that of pollens and other allergens.3
Quito, the capital of Ecuador, is a city located in the Andes Mountains, at 2,800m above sea level. Its temperature ranges from 7°C at night, to 26°C during the day, averaging 15°C , and its annual mean relative humidity is 75%. While children's greatest exposure to indoor allergens is in the home, other public places where children spend a large amount of time, such as schools, may also be sources of significant allergen encounters. Studies on the significance of environmental conditions and dust mites concentrations in the school environment are scarce in the world, and absent in Ecuador and many other Latin-American countries. Students spend a great deal of their time in the classroom, and aeroallergen exposure in the school environment could facilitate allergic sensitisation and subsequent development of asthma.
To determine asthma prevalence and characteristics, sensitisation and exposure levels to Der p 1 and Der f 1 in students of Quito, we researched an urban private elementary school with 278 students. One hundred and ten students participated in the study. Their age ranged from 6.3 to 20.7 years with a mean of 12.3 years. Forty-seven (42.7%) were males and 63 (57.2%) females.
Every child was given a complete physical examination and PEFR (ASSESS portable device, Health Scan Products inc, NJ, USA). Skin prick tests were conducted on the volar surface of the forearms using a standard battery of skin tests, including standardised extracts of D. pteronyssinus,D. farinae,Lepidoglyphus destructor, Tyrophagus putrescentiae, grasses mix (Dactylis glomerata,Festuca pratensis,Poa pratensis,Phleum pratensis, Lolium perenne), weeds mix (Artemisia vulgaris, Chenopodium album, Parietaria judaica, Plantago lanceolata, Taraxacum officinale), dog and cat dander and mould mix (Alternaria tenuis, Chaetomium globosum, Cladosporium fulvum, C.herbarum, Fusarium sp, Aspergilus amstelodami, A. fumigatus, A. niger, A. terreus) (Laboratorios LETI, Tres Cantos, Madrid, Spain). Written questionnaires were sent to the student's parents inquiring about the presence of asthma or any chronic bronchial and allergic diseases displayed by the student or close relatives, and the smoking habits of the parents. Dust samples were collected from 10 classrooms and analysed for Der p 1 and Der f 1 allergens.
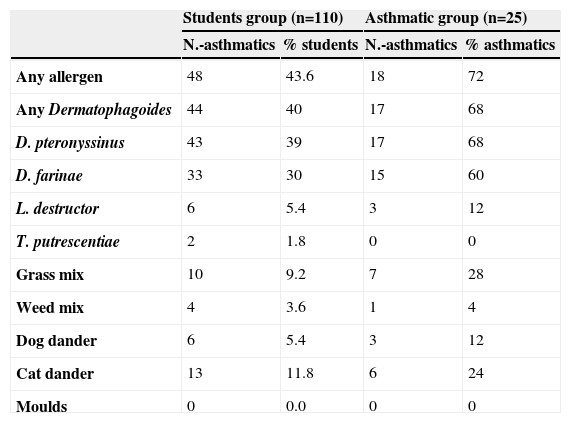
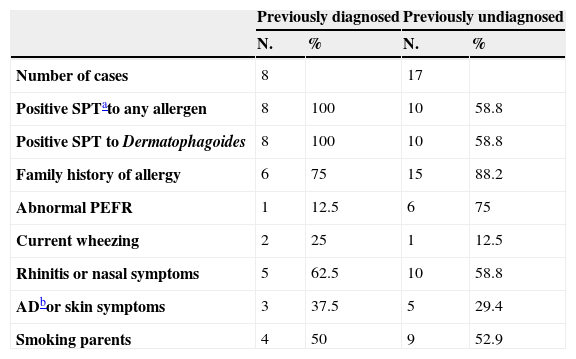
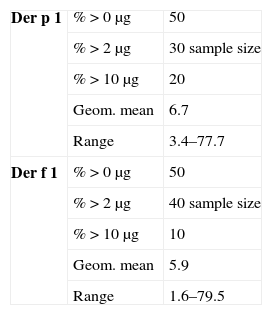
Out of the 110 students tested, 48 (43.6%) had skin sensitisations to at least one allergen. The main sensitiser was Dermatophagoides with 43 (39.0%) sensitised to D. pteronyssinus, and 33 (30.0%) to D. farinae (Table 1). Parents of 99 students responded to the questionnaires and among those students we found 25 (25.2%) asthmatics (8 previously diagnosed by a physician and 17 previously undiagnosed). Table 2 shows the clinical characteristics of the 25 asthmatic students. Twenty-one (84%) of the 25 asthmatics had a family history of allergies, and 18 (72%) had skin tests positive to at least one allergen. Also Dermatophagoides was the main allergen among asthmatics, with 17 (68%) of them sensitised to D. pteronyssinus and 15 (60%) to D. farinae. Differences were not significant comparing the asthmatics and their sensitisation to D. pteronyssinus, in any age or sex group. The other allergens tested produced statistical lesser sensitisations than those obtained with Dermatophagoides, in the group of 110 students and in the group of 25 asthmatic children. Seven (28%) of the 25 asthmatic students presented a PEFR lower to the 80% of the predicted values and three (12%) presented current wheezing upon physical examination. We have not found a relationship between asthma and parental smoking. High Levels of Der p 1 (geometric mean of 6.76ug/g, range from 3.4 to 77.7) and Der f 1 (5.93ug/g, range from 1.6 to 79.5) were found in 50% of the dust samples collected (Table 3). In most cases allergen levels exceeded the concentrations which have been proposed for sensitisation and presence of symptoms.4 In similar studies, detectable but lower levels of dust allergens were reported by North American authors.5,6
Skin sensitisation rates in the 110 students¿
| Students group (n=110) | Asthmatic group (n=25) | |||
| N.-asthmatics | % students | N.-asthmatics | % asthmatics | |
| Any allergen | 48 | 43.6 | 18 | 72 |
| Any Dermatophagoides | 44 | 40 | 17 | 68 |
| D. pteronyssinus | 43 | 39 | 17 | 68 |
| D. farinae | 33 | 30 | 15 | 60 |
| L. destructor | 6 | 5.4 | 3 | 12 |
| T. putrescentiae | 2 | 1.8 | 0 | 0 |
| Grass mix | 10 | 9.2 | 7 | 28 |
| Weed mix | 4 | 3.6 | 1 | 4 |
| Dog dander | 6 | 5.4 | 3 | 12 |
| Cat dander | 13 | 11.8 | 6 | 24 |
| Moulds | 0 | 0.0 | 0 | 0 |
Clinical characteristics of the 25 asthmatic students
| Previously diagnosed | Previously undiagnosed | |||
| N. | % | N. | % | |
| Number of cases | 8 | 17 | ||
| Positive SPTato any allergen | 8 | 100 | 10 | 58.8 |
| Positive SPT to Dermatophagoides | 8 | 100 | 10 | 58.8 |
| Family history of allergy | 6 | 75 | 15 | 88.2 |
| Abnormal PEFR | 1 | 12.5 | 6 | 75 |
| Current wheezing | 2 | 25 | 1 | 12.5 |
| Rhinitis or nasal symptoms | 5 | 62.5 | 10 | 58.8 |
| ADbor skin symptoms | 3 | 37.5 | 5 | 29.4 |
| Smoking parents | 4 | 50 | 9 | 52.9 |
In conclusion, there is a considerable proportion of dust mite allergic school children in Quito. Dust mite allergens may be present in schools in Quito in concentrations to induce allergic/asthmatic symptoms. The humidity of 75% in spite of the height of 2,800m above sea level may explain the presence of dust mites.